Abstract
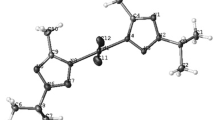
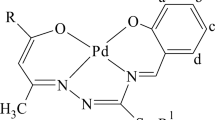
The reactions of palladium(II) chloride with catecholamines (hydrochlorides of 3-methoxytyramine, normetanephrine, norepinephrine, and dopamine) and pyridine-2-carbaldehyde have afforded four new palladium complexes of different types (pyridine-imine, oxazolidine-imine, and cation-anionic ones). Structure of the obtained complexes have been confirmed by means of NMR spectroscopy and X-ray diffraction analysis. Cytotoxic activity of the complexes with respect to PC3 (prostate cancer cells) and HEK-293 (human epithelial kidney cells) cell lines has been estimated.






Similar content being viewed by others
REFERENCES
Denisov, M.S. and Glushkov, V.A., Vestn. Permsk. Univ., Ser. Khim., 2018, vol. 4, no. 4, p. 388. https://doi.org/10.17072/2223-1838-2018-4-388-411
Serratrice, M., Maiore, L., Zucca, A., Stoccoro, S., Landini, I., Mini, E., Massai, L., Ferraro, G., Merlino, A., Messori, L., and Cinellu, M.A., Dalton Trans., 2016, vol. 45, p. 579. https://doi.org/10.1039/C5DT02714D
Mitra, I., Mukherjee, S., Reddy, B.V.P., Misini, B., Das, P., Dasgupta, S., Linert, W., and Moi, S.Ch., New J. Chem., 2018, vol. 42, p. 2574. https://doi.org/10.1039/C7NJ05173E
Egorova, K.S., Galushko, A.S., and Ananikov, V.P., Angew. Chem. Int. Ed., 2020, vol. 59, p. 22296. https://doi.org/10.1002/anie.202003082
Valentini, A., Conforti, F., Crispini, A., Martino, A.D., Condello, R., Stellitano, C., Rotilio, G., Ghedini, M., Federici, G., Bernardini, S., and Pucci, D., J. Med. Chem., 2009, vol. 52, no. 2, p. 484. https://doi.org/10.1021/jm801276a
Büyükekşi, S.I., Erkısa, M., Şengül, A., Ulukaya, E., and Oral, A.Y., Appl. Organometal. Chem., 2018, vol. 32, no. 8, p. e4406. https://doi.org/10.1002/aoc.4406
Ulukaya, E., Ari, F., Dimas, K.,·Sarimahmut, M., Guney, E., Sakellaridis, N.,·and Yilmaz, V.T., J. Cancer Res. Clin. Oncol., 2011, vol. 137, p. 1425. https://doi.org/10.1007/s00432-011-1021-1
Denisov, M.S. and Glushkov, V.A., Russ. Chem. Bull., 2020, vol. 69, no. 10, p. 2013. https://doi.org/10.1007/s11172-020-2993-2
Gonçalves, B.M.F., Salvador, J.A.R., Marín, S., and Cascante, M., Eur. J. Med. Chem., 2016, vol. 114, p. 101. https://doi.org/10.1016/j.ejmech.2016.02.057
Haribabu, J., Srividya, S., Mahendiran, D., Gayathri, D., Venkatramu, V., Bhuvanesh, N., and Karvembu, R., Inorg. Chem., 2020, vol. 59, no. 23, p. 17109. https://doi.org/10.1021/acs.inorgchem.0c02373
Gichumbi, J.M., Friedrich, H.B., Omondi, B., Singh, M., Naidoo, K., and Chenia, H.Y., J. Coord. Chem., 2016, vol. 69, no. 23, p. 3531. https://doi.org/10.1080/00958972.2016.1243238
Reddy, E.R., Trivedi, R., Sarma, A.V.S., Sridhar, B., Anantaraju, H.Sh., Sriram, D., Yogeeswarid, P., and Nagesh, N., Dalton Trans., 2015, vol. 44, p. 17600. https://doi.org/10.1039/C5DT03266K
Denisov, M.S., Gagarskih, O.N., and Utushkina, T.A., Vestn. Permsk. Univ., Ser. Khim., 2021, vol. 11, no. 1, p. 30. https://doi.org/10.17072/2223-1838-2021-1-30-58
Striegler, S. and Dittel, M., Inorg. Chem., 2005, vol. 44, no. 8, p. 2728. https://doi.org/10.1021/ic048724p
Molaeea, H., Moghadama, M., Mirkhania, V. Tangestaninejada, Sh., Mohammadpoor-Baltorka, I., Kajania, A.A., and Kia, R., Polyhedron., 2019, vol. 160, no. 1, p. 130. https://doi.org/10.1016/j.poly.2018.11.037
Motswainyana, W.M., Onani, M.O., Madiehe, A.M., Saibu, M., Jacobs, J., and van Meervelt, L., Inorg. Chimica Acta, 2013, vol. 400, no. 1, p. 197. https://doi.org/10.1016/j.ica.2013.02.029
Shaidarova, L.G., Chelnokova, I.A., Leksina, Y.A., Gedmina, A.V., and Budnikov, H.C., J. Anal. Chem., 2020, vol. 75, no. 8, p. 1059. https://doi.org/10.1134/S1061934820080134
Ossola, B., Schendzielorz, N., Chen, Sh.-H., Bird, G.S., Tuominen, R.K., Männistö, P.T., and Hong, J.-Sh., Neuropharmacology, 2011, vol. 61, no. 4, p. 574. https://doi.org/10.1016/j.neuropharm.2011.04.030
Zalevskaya, O.A., Gur’eva, Ya.A., and Kutchin, A.V., Russ. Chem. Rev., 2019, vol. 88, no. 10, p. 979. https://doi.org/10.1070/RCR4880
Denisov, M.S., Dmitriev, M.V., Eroshenko, D.V., Slepukhin, P.A., Shavkunov, S.P., and Glushkov, V.A., Russ. J. Inorg. Chem., 2019, vol. 64, no. 1, p. 56. https://doi.org/10.1134/S0036023619010054
CrysAlisPro, Agilent Technologies, Version 1.171.37.33 (release 27-03-2014 CrysAlis171 .NET).
Sheldrick, G.M., Acta Crystallogr. (A), 2015, vol. 71, p. 3. https://doi.org/10.1107/S2053273314026370
Sheldrick, G.M., Acta Crystallogr. (C), 2015, vol. 71, p. 3. https://doi.org/10.1107/S2053229614024218
Dolomanov, O.V., Bourhis, L.J., Gildea, R.J., Howard, J.A.K., and Puschmann, H., J. Appl. Cryst., 2009, vol. 42, p. 339. https://doi.org/10.1107/S0021889808042726
Mercury 3.3 (Build RC5). Cambridge: Cambridge Crystallographic Data Centre, 2013. http://www.ccdc.cam.ac.uk/mercury/
ACKNOWLEDGMENTS
The work was carried out using the equipment of The Core Facilities Center “Research of materials and matter” at the Perm Federal Research Center, Ural Branch, RAS. Authors are grateful to O.A. Mayorova (Institute of Technical Chemistry of Ural Branch, Russian Academy of Sciences) for recording the NMR spectra, D.K. Trukhinov and D.M. Kiselkov (Institute of Technical Chemistry of Ural Branch, Russian Academy of Sciences) for recording the IR spectra, M.V. Dmitriev (Perm State National Research University) for the X-ray diffraction analysis, T.E. Oschepkova (Institute of Technical Chemistry of Ural Branch, Russian Academy of Sciences) for performing the thermogravimetric analysis, S.P. Shavkunov (Perm State National Research University) for performing the conductometry measurements, and Yu.А. Beloglazova and A.O. Voronina (Institute of Technical Chemistry of Ural Branch, Russian Academy of Sciences) for the assistance in performing the MTT test.
Funding
The reported study was funded by Russian Foundation for Basic Research and Ministry of Education and Science of Perm District according to the research project no. 19-43-590003.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
No conflict of interest was declared by the authors.
Additional information
Translated from Zhurnal Obshchei Khimii, 2021, Vol. 91, No. 7, pp. 1092–1099 https://doi.org/10.31857/S0044460X21070131.
Rights and permissions
About this article
Cite this article
Denisov, M.S., Gagarskikh, O.N. Palladium(II) Complexes with Catecholamines: Synthesis and In Vitro Cytotoxic Activity. Russ J Gen Chem 91, 1354–1360 (2021). https://doi.org/10.1134/S1070363221070136
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070363221070136