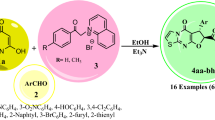
Abstract
The sulfa-Michael/aldol cascade reaction of (Z)-7-arylidene -3-aryl-3,4-dihydro-2H-thiazolo[3,2-a][1,3,5]triazin-6(7H)-one with 1,4-dithiane-2,5-diol leads to novel 4ʹ-hydroxy-2ʹ,3-diaryl-3,4,4ʹ,5ʹ-tetrahydro-2H,2ʹH,6H-spiro[thiazolo[3,2-a][1,3,5]triazine-7,3ʹ-thiophen]-6-ones in moderate yields. Structures of all the products are characterized by NMR, IR, and HRMS spectra in combination with X-ray crystallographic analysis.



Similar content being viewed by others
REFERENCES
Klenke, B., Stewart, M., Barrett, M.P., Brun, R., and Gilbert, I.H., J. Med. Chem., 2001, vol. 44, p. 3440. https://doi.org/10.1021/jm010854+
Maeda, S., Kita, T., and Meguro, K., J. Med. Chem., 2009, vol. 52, p. 597. https://doi.org/10.1021/jm8014712
Nishigaki, S., Yoneda, F., Matsumoto, H., and Morinaga, K., J. Med. Chem., 1969, vol. 12, p. 39. https://doi.org/10.1021/jm00301a010
Rees, R.W.A., Russell, P.B., Foell, T.J., and Bright, R.E., J. Med. Chem., 1972, vol. 15, p. 859. https://doi.org/10.1021/jm00278a024
Li, G., Meng, X., Wang, J., Wang, Q., Zhou, J., Wang, C., Wu, Q., and Wang, Z., Food Chem., 2020, vol. 309, p. 125618. https://doi.org/10.1016/j.foodchem.2019.125618
Do, K., Choi, H., Lim, K., Jo, H., Cho, J., Nazeeruddin, M.K., and Ko, J., Chem. Commun., 2014, vol. 50, p.10971. https://doi.org/10.1039/C4CC04550E
Wang, J., Ouyang, G., Wang, Y., Qiao, X., Li, W., and Li, H., Chem. Commun., 2020, vol. 56, p. 1601. https://doi.org/10.1039/C9CC08412F
Yoshikawa, M., Morikawa, T., Matsuda, H., Tanabe, G., and Muraoka, O., Bioorg. Med. Chem., 2002, vol. 10, p. 1547. https://doi.org/10.1016/S0968-0896(01)00422-9
Haraguchi, K., Shimada, H., Tanaka, H., Hamasaki, T., Baba, M., Gullen, E.A., Dutschman, G.E., and Cheng, Y.C., J. Med. Chem., 2005, vol. 51, p. 1885. https://doi.org/10.1021/jm070824s
Jeong, L.S., Choe, S.A., Gunaga, P., Kim, H.K., Lee, H.W., Lee, S.K., Tosh, D.K., Patel, A., Palaniappan, K.K., Gao, Z., Jacobson, K.A., and Moon, H.R., J. Med. Chem., 2007, vol. 50, p. 3159. https://doi.org/10.1021/jm070259t
Volkmann, R.A., Kelbaugh, P.R., Nason, D.M., and Jasys, V.J., J. Org. Chem., 1992, vol. 57, p. 4352. https://doi.org/10.1021/jo00042a010
Qiao, C., Ling, K., Shepard, E.M., Dooley D.M., and Sayre, L.M., J. Am. Chem. Soc., 2006, vol. 128, p. 6206. https://doi.org/10.1021/ja058838f
Chauhan, P., Mahajan, S., and Enders, D., Chem. Rev., 2014, vol. 114, p. 8807. https://doi.org/10.1021/cr500235v
Duan, S., Li, Y., Liu, Y.Y., Zou, Y.Q., Shi, D.Q., and Xiao, W.J., Chem. Commun., 2012, vol. 48, p.5160. https://doi.org/10.1039/C2CC30931A
Kowalczyk, D., Wojciechowski, J., and Albrecht, L., Tetrahedron Lett., 2016, vol. 57, p. 2533. https://doi.org/10.1016/j.tetlet.2016.04.111
Kumara, C.S.P., Gowda, G.B., Kumar, K.S.V., Ramesh, N., Sadashiva, M.P., and Junjappa, H., Tetrahedron Lett., 2016, vol. 57, p. 4302. https://doi.org/10.1016/j.tetlet.2016.08.033
Nagaraju, S., Sathish, K., Paplal, B., and Kashinath, D., Tetrahedron Lett. 2017,vol. 58, p. 2865. https://doi.org/10.1016/j.tetlet.2017.06.029
Yang, L., Li, X., Hu, X., Yu, X., Tetrahedron Lett., 2016, vol. 57, p. 1265. https://doi.org/10.1016/j.tetlet.2016.02.020
Liu, B., Li, X., Liu, H., Yu, X., Tetrahedron Lett., 2013, vol. 54, p. 6952. https://doi.org/10.1016/j.tetlet.2013.10.062
Fu, X.L., Meng, Y.K., Li, X., Stępień, M., and Chmielewski, P.J., Chem. Commun., 2018, vol. 54, p. 2510. https://doi.org/10.1039/c8cc00447a
Ren, D., Koniarz, S., Li, X., Chmielewski, P., Chem. Commun., 2020, vol. 56, p. 4836. https://doi.org/10.1039/d0cc01013h
ACKNOWLEDGMENTS
We are grateful to the National Natural Science Foundation of China (No. 21671063) for the financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
No conflict of interest was declared by the authors.
Rights and permissions
About this article
Cite this article
Li, S., Tan, J. & Li, X. Synthesis of Novel 4ʹ-Hydroxy-2ʹ,3-diaryl-3,4,4ʹ,5ʹ-tetrahydro-2H,2ʹH,6H-spiro[thiazolo[3,2-a][1,3,5]triazine-7,3ʹ-thiophen]-6-one Derivatives via Sulfa-Michael/Aldol Cascade Reactions. Russ J Gen Chem 91, 128–132 (2021). https://doi.org/10.1134/S1070363221010151
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070363221010151