Abstract
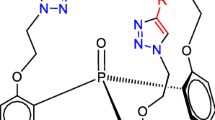
New tripodal 1,2,3-triazole ligands based on triphenylphosphine oxide platform with different linker lengths have been synthesized, and their structure has been determined by elemental analysis, 1H, 13C, and 31P NMR spectroscopy, mass spectrometry, and vibrational (IR and Raman) spectroscopy. The coordination and extraction properties of the new ligands have been studied in the complexation with uranyl nitrate and extraction of micro amounts of uranium(VI) from aqueous phase to 1,2-dichloroethane.





Similar content being viewed by others
REFERENCES
Aromí, G., Barrios, L.A., Roubeau, O., and Gamez, P., Coord. Chem. Rev., 2011, vol. 255, p. 485. https://doi.org/10.1016/j.ccr.2010.10.038
Schulze, B. and Schubert, U.S., Chem. Soc. Rev., 2014, vol. 43, p. 2522. https://doi.org/10.1039/c3cs60386e
Götzke, L., Schaper, G., März, J., Kaden, P., Huittinen, N., Stumpf, T., Kammerlander, K.K.K., Brunner, E., Hahn, P., Mehnert, A., Kersting, B., Henle, T., Lindoy, L.F., Zanoni, G., and Weigand, J.J., Coord. Chem. Rev., 2019, vol. 386, p. 267. https://doi.org/10.1016/j.ccr.2019.01.006
Kudryavtsev, I.Yu., Baulina, T.V., Pasechnik, M.P., Matveev, S.V., and Matveeva, A.G., Phosphorus, Sulfur Silicon Relat. Elem., 2014, vol. 189, nos. 7–8, p. 946. https://doi.org/10.1080/10426507.2014.904865
Matveeva, A.G., Kudryavtsev, I.Yu., Pasechnik, M.P., Vologzhanina, A.V., Baulina, T.V., Vavina, A.V., Sukat, G.Ya., Matveev, S.V., Godovikov, I.A., Turanov, A.N., Karandashev, V.K., and Brel, V.K., Polyhedron, 2018, vol. 142, p. 71. https://doi.org/10.1016/j.poly.2017.12.025
Bykhovskaya, O.V., Matveeva, A.G., Pasechnik, M.P., Vologzhanina, A.V., Matveev, S.V., Kudryavtsev, I.Yu., Baulina, T.V., and Brel, V.K., Russ. J. Gen. Chem., 2019, vol. 89, no. 12, p. 2400. https://doi.org/10.1134/S1070363219120120
Kudryavtsev, I.Yu., Bykhovskaya, O.V., Matveeva, A.G., Baulina, T.V., Pasechnik, M.P., Matveev, S.V., Vologzhanina, A.V., Turanov, A.N., Karandashev, V.K., and Brel, V.K., Monatsh. Chem., 2020, vol. 151, p. 1705. https://doi.org/10.1007/s00706-020-02702-6
Jones, M.B. and Gaunt, A.J., Chem. Rev., 2013, vol. 113, p. 1137. https://doi.org/10.1021/cr300198m
Carter, K.P. and Cahill, C.L., Inorg. Chem. Front., 2015, vol. 2, p. 141. https://doi.org/10.1039/c4qi00183d
Mazzanti, M., Wietzke, R., Pécaut, J.,. Latour, J.-M, Maldivi, P., and Remy, M., Inorg. Chem., 2002, vol. 41, p. 2389. https://doi.org/10.1021/ic010839v
Van Horn, J.D. and Huang, H., Coord. Chem. Rev., 2006, vol. 250, p. 765. https://doi.org/10.1016/j.ccr.2005.09.0
Urankar, D., Pevec, A., Turel, I., and Košmrlj, J., Cryst. Growth Des., 2010, vol. 10, no. 11, p. 4920. https://doi.org/10.1021/cg100993k
Nakamoto, K., Infrared and Raman Spectra of Inorganic and Coordination Compounds, Hoboken: Wiley, 2009, 6th ed.
Laikov, D.N., Chem. Phys. Lett., 1997, vol. 281, p. 151. https://doi.org/10.1016/S0009-2614(97)01206-2
Laikov, D.N., Chem. Phys. Lett., 2005, vol. 416, p. 116. https://doi.org/10.1016/j.cplett.2005.09.046
Laikov, D.N. and Ustynyuk, Yu.N., Russ. Chem. Bull., Int. Ed., 2005, vol. 54, p. 820. https://doi.org/10.1007/s11172-005-0329-x
Szabó, Z., Toraishi, T., Vallet, V., and Grenthe, I., Coord. Chem. Rev., 2006, vol. 250, p. 784. https://doi.org/10.1016/j.ccr.2005.10.005
Redmond, M.P., Cornet, S.M., Woodall, S.D., Whittaker, D., Collison, D., Helliwell, M., and Natrajan, L.S., Dalton Trans., 2011, vol. 40, p. 3914. https://doi.org/10.1039/c0dt01464h
Wahu, S., Berthet, J.-C., Thuéry, P., Guillaumont, D., Ephritikhine, M., Guillot, R., Cote, G., and Bresson, C., Eur. J. Inorg. Chem., 2012, vol. 23, p. 3747. https://doi.org/10.1002/ejic.201200243
Häller, L.J.L., Kaltsoyannis, N., Sarsfield, M.J., May, I., Cornet, S.M., Redmond, M.P., and Helliwell, M., Inorg. Chem., 2007, vol. 46, no. 12, p. 4868. https://doi.org/10.1021/ic062031m
Colasson, B., Le Poul, N., Le Mest, Y., and Reinaud, O., Inorg. Chem., 2011, vol. 50, p. 10985. https://doi.org/10.1021/ic201540x
Das, D., Kannan, S., Maity, D.K., and Drew, M.G.B., Inorg. Chem., 2012, vol. 51, p. 4869. https://doi.org/10.1021/ic300398a
Armarego, W.L.F. and Chai, C.L.L., Purification of Laboratory Chemicals, New York: Elsevier, 2009, p 743. https://doi.org/10.1134/S0044460X1809024X
Gel’man, N.E., Terent’eva, E.A., Shanina, T.M., and Kiparenko, L.M., Metody kolichestvennogo organicheskogo elementnogo mikroanaliza (Methods of Quantitative Organoelement Microanalysis), Moscow: Khimiya, 1987.
Kudryavtsev, I.Yu., Bykhovskaya, O.V., Aladzheva, I.M., Baulina, T.V., and Brel, V.K., Russ. J. Gen. Chem., 2017, vol. 87, no. 11, p. 2744. https://doi.org/10.1134/S1070363217110366
Baulina, T.V., Kudryavtsev, I.Yu., Sukat, G.Ya., and Brel, V.K., Russ. J. Gen. Chem., 2018, vol. 88, no. 9, p. 1927. https://doi.org/10.1134/S1070363218090281
Baulina, T.V., Kudryavtsev, I.Yu., Smolyakov, A.F., Pasechnik, M.P., and Brel, V.K., Heteroat. Chem., 2018, vol. 29, p. e21454. https://doi.org/10.1002/hc.21454
Turanov, A.N., Karandashev, V.K., Baulin, V.E., and Tsvetkov, E.N., Russ. J. Inorg. Chem., 1995, vol. 40, p. 1926.
Funding
TThis work was financially supported by the Russian Science Foundation (project no. 20–13–00329). Spectral studies were carried out using the equipment of the Center for Molecular Structure Studies, Nesmeyanov Institute of Organoelement Compounds, Russian Academy of Sciences.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare the absence of conflict of interest.
Rights and permissions
About this article
Cite this article
Matveeva, A.G., Baulina, T.V., Kudryavtsev, I.Y. et al. Tripodal 1,2,3-Triazole Ligands Based on Triphenylphosphine Oxide. Coordination and Extraction Properties. Russ J Gen Chem 90, 2338–2349 (2020). https://doi.org/10.1134/S107036322012018X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S107036322012018X