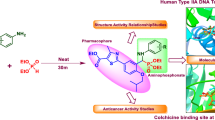
Abstract
A new four-components post-Ugi transformation process has been studied. It provides an efficient access to biologically active piperazine-2,5-dione derivatives in high yield. The framework of piperazine-2,5-dione derivatives has been constructed by a tandem-decarboxylation of α-keto carboxylic acids promoted by a green catalyst trimethylsilyl trifluoromethane sulfonate (TMSOTf). Molecular docking study of piperazine-2,5-dione derivatives has been performed with various anticancer target proteins: human androgen receptor (AR) (PDB ID: 1E3G), human steroidogenic cytochrome P450 17A1 (PDB ID: 4NKV), epidermal growth factor receptor 2 HER2 (PDB ID: 3PP0), and estrogen receptor alpha (ERα) (PDB ID: 1A52), and has indicated their possible efficient interactions via hydrogen bonds.




Similar content being viewed by others
REFERENCES
Ressurreição, S.M., Delatouche, R., Gennari, C., and Piarulli, U., Eur. J. Org. Chem., 2011, vol. 2011, p. 217. https://doi.org/10.1002/ejoc.201001330
Yamazaki, Y., Sumikura, M., Masuda, Y., Hayashi, Y., Yasui, H., Kiso, Y., Chinen, T., Usui, T., Yakushiji, F., Potts, B., Neuteboom, S., Palladino, M., Lloyd, G.K., and Hayashi, Y., Bioorg. Med. Chem., 2012, vol. 20, p. 4279. https://doi.org/10.1016/j.bmc.2012.05.059.
Zhao, S., Smith, K.S., Deveau, A.M., Dieckhaus, C.M., Johnson, M.A., Macdonald, T.L., and Cook, J.M., J. Med. Chem., 2002, vol. 45, p. 1559. https://doi.org/10.1021/jm0155953
Hernández-Vázquez, E., and Miranda, L.D., Org. Biomo. Chem., 2016, vol. 14, p. 4875. https://doi.org/10.1039/C6OB00431H
González, J.F., Ortín, I., de la Cuesta, E., and Menéndez, J.C., Chem. Soc. Rev., 2012, vol. 41, p. 6902. https://doi.org/10.1039/C2CS35158G
Jassem, A.M., Almashal, F.A.K., Mohammed, M.Q., and Jabir, H.A.S., SN. Appl. Sci., 2020, vol. 2, p. 359. https://doi.org/10.1007/s42452-020-2165-x
Jassem, A.M., Almashal, F.A.K., and Jaber, H.A.S., Russ. J. Gen. Chem., 2020, vol. 90, p. 895. https://doi.org/10.1134/S1070363220050230
Jassem, A.M., Al-Ajely, H.M., Almashal, F.A.K., and Chen, B., Russ. J. Gen. Chem., 2019, vol. 89, p. 2562. https://doi.org/10.1134/S1070363219120363
Ribelin, T.P., Judd, A.S., Akritopoulou-Zanze, I., Henry, R.F., Cross, J.L., Whittern, D.N., and Djuric, S.W., Org. Lett., 2007, vol. 9, p. 5119. https://doi.org/10.1021/ol7023373
Marcaccini, S., Pepino, R., Pozo, M.C., Basurto, S., Garcı́a-Valverde, M., and Torroba, T., Tetrahedron. Lett., 2004, vol. 45, p. 3999. https://doi.org/10.1016/j.tetlet.2004.03.184
Tyagi, V., Khan, S., and Chauhan, P.M., Synlett., 2013, vol. 24, p. 1291. https://doi.org/10.1055/s-0033-1338707
Wright, D.L., Robotham, C.V., and Aboud, K., Tetrahedron Lett., 2002, vol. 43, p. 943. https://doi.org/10.1016/S0040-4039(01)02299-7
Corres, N., Delgado, J.J., García-Valverde, M., Marcaccini, S., Rodríguez, T., Rojo, J., and Torroba, T., Tetrahedron., 2008, vol. 64, p. 2225. https://doi.org/10.1016/j.tet.2007.12.028
Xu, Z., De Moliner, F., Cappelli, A.P., and Hulme, C., Org. Lett. 2013, vol. 15, p. 2738. https://doi.org/10.1021/ol401068u
Azuaje, J., Pérez-Rubio, J.M., Yaziji, V., El Maatougui, A., González-Gomez, J.C., Sánchez-Pedregal, V.M., NavarroVázquez, A., Masaguer, C.F., Teijeira, M., and Sotelo, E., J. Org. Chem., 2015, vol. 80, p. 1533. https://doi.org/10.1021/jo502382q
Polindara-García, L.A., and Vazquez, A., Org. Biomo. Chem., 2014, vol. 12, p. 7068. https://doi.org/10.1039/C4OB00767K
VenkataPrasad, J., Krishnamurthy, S., Moriguchi, T., and Tsuge, A., New. J. Chem., 2017, vol. 41, p. 97. https://doi.org/10.1039/C6NJ02569B.
Miranda, L.D., and Hernández-Vázquez, E., J. Org. Chem., 2015, vol. 80, p. 10611. https://doi.org/10.1021/acs.joc.5b01742
Yan, Y.M., Gao, Y., and Ding, M.W., Tetrahedron., 2016, vol. 72, p. 5548. https://doi.org/10.1016/j.tet.2016.07.048
Li, Z., Kumar, A., Sharma, S.K., Parmar, V.S., and Van der Eycken, E.V., Tetrahedron., 2015, vol. 71, p. 3333. https://doi.org/10.1016/j.tet.2015.03.103
Jassem, A.M., Dhumad, A.M., Almashal, F.A., and Alshawi, J.M., Med. Chem. Res., 2020, vol. 29, p. 1067. https://doi.org/10.1007/s00044-020-02546-z.
Lee, C., Yang, W., and Parr, R.G., Physical. Rev. B., 1988, vol. 37, p. 785. https://doi.org/10.1103/PhysRevB.37.785
Trott, O., and Olson, A.J., J. Comput. Chem., 2010, vol. 31, p. 455. https://doi.org/10.1002/jcc.21334
Yuan, S., Chan, H.S., and Hu, Z., Wiley. Inter. Rev: Comput. Mole. Sci., 2017, vol. 7, p. 1298. https://doi.org/10.1002/wcms.1298
Capaia, M., Granata, I., Guarracino, M., Petretto, A., Inglese, E., Cattrini, C., Ferrari, N., Boccardo, F., and Barboro, P., Inter.J. Mole. Sci., 2018, vol. 19, p. 1920. https://doi.org/10.3390/ijms19071920
ACKNOWLEDGMENTS
Authors gratefully thank Leicester University, UK for performing 1H, 13C NMR, FTIR, HRMS spectra, and HPLC analysis.
Funding
This work was financially supported by the authors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
No conflict of interest was declared by the authors.
Rights and permissions
About this article
Cite this article
Jassem, A.M., Dhumad, A.M. & Almashal, F.A.K. Synthesis of New Drug-Like Piperazine-2,5-diones by the Ugi/Tandem Process Catalyzed by TMSOTf and Their Molecular Docking. Russ J Gen Chem 90, 2181–2188 (2020). https://doi.org/10.1134/S1070363220110262
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070363220110262