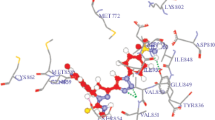
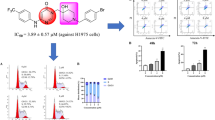
Abstract
A novel series of imidazo [1,2-a]pyridine derivatives has been designed, synthesized and tested for the anti-proliferative activity against three different human cancer cell lines. Most of the synthesized compounds exhibit anti-proliferative activity with IC50 values ranging from 5.35–59.8 μM. Six compounds demonstrate efficient inhibition of growth of all cell lines with IC50 values close to that of standard drug, and the compound 16h is more potent than the standard drug cisplatin for the HeLa cell line.



Similar content being viewed by others
REFERENCES
Couty, F. and Evano, G., in Comprehensive Heterocyclic Chemistry III, Katritzky, A.R., Ramsden, C.A., Scriven, E.F.V., and Taylor, R.J.K., Eds., Oxford: Elsevier, 2008, vol. 11, p. 409.
Rupert, K.C., Henry, J.R., Dodd, J.H., Wadsworth, S.A., Cavender, D.E., Olini, G.C., Fahmy, B., and Siekierka, J., Bioorg. Med. Chem. Lett., 2003, vol. 13, p. 347. https://doi.org/10.1016/s0960-894x(02)01020-x
Hranjec, M., Kralj, M., Piantanida, I., Sedi, M., Suman, L., Pavel, K., and Karminski-Zamola, G., J. Med. Chem., 2007, vol. 50, p. 5696. https://doi.org/10.1021/jm070876h
Kotovskaya, S.K., Baskakova, Z.M., Charushin, V.N., Chupakhin, O.N., Belanov, E.F., Bormotov, N.I., Balakhnin, S.M., and Serova, O.A.,Pharm. Chem. J., 2005, vol. 39, p. 574. https://doi.org/10.1007/s11094-006-0023-9
Lhassani, M., Chavignon, O., Chezal, J.M., Teulade, J.C., Chapat, J.P., Snoeck, R., Andrei, G., Balzarini, J., Clerc, E.D., and Gueiffier, A.,Eur. J. Med. Chem., 1999, vol. 34, p. 271. https://doi.org/10.1016/S0223-5234(99)80061-0
Langer, S.Z., Arbilla, S., Benavides, J., and Scatton, B.,Adv. Biochem. Psychopharmacol, 1990, vol. 46, p. 61. PMID: 1981304.
Mizushige, K., Ueda, T., Yukiiri, K., and Suzuki, H., Cardiovasc. Drug Rev., 2002, vol. 20, p. 163. https://doi.org/10.1111/j.1527-3466.2002.tb00085.x
Almirante, L., Polo, L., Mugnaini, A., Provinciali, E., Rugarli, P., Biancotti, A., Gamba, and Murmann, W., J. Med. Chem., 1965, vol. 8, p. 305. https://doi.org/10.1021/jm00327a007
Boerner, R.J. and Moller, H.J., Psychopharmakother, 1997, vol. 4, p. 145.
Li, H.Q., Lv, P.C., Yan, T., and Zhu, H.L., Anticancer Agents Med. Chem., 2009, vol. 10, p. 471. https://doi.org/10.2174/1871520610909040471
Wilhelm, S.M., Carter, C., Tang, L., Wilkie, D., McNabola, A., Rong, H., Chen, C., Zhang, X., Vincent, P., Mchugh, M., Cao, Y., Shujath, J., Gawlak, S., Eveleigh, D., Rowley, B., Liu, L., Adnane, L., Lynch, M., Auclair, D., Taylor, I., Gedrich, R., Voznesensky, A., Riedl, B., Post, L.E., and Bollag, G., Cancer Res., 2004, vol. 64, p. 7099. https://doi.org/10.1158/0008-5472
Battaglia, E., Bagrel, D., Migianu, E., Brault, L., Néguesque, A., Kirsch, G., Eur. J. Med Chem., 2005, vol. 40, p. 757. https://doi.org/10.1016/j.ejmech.2005.02.010
Ye, D., Zhang, Y., Wang, F., Zheng, M., Zhang, X., Luo, X., Shen, X., Jiang, H., and Liu, H., Bioorg. Med. Chem., 2010, vol. 18, p. 1773. https://doi.org/10.1016/j.bmc.2010.01.055
ACKNOWLEDGMENTS
The authors would like to thank the management of AMRI Hyderabad research centre for giving an opportunity to carry out this research. The authors are also thankful to SaiInnotech labs Pvt.Ltd.for providing synthesis facility.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
No conflict of interest was declared by the authors.
Rights and permissions
About this article
Cite this article
Endoori, S., Gulipalli, K.C., Bodige, S. et al. Design, Synthesis, Anti-Cancer Activity, and in silico Studies of Novel Imidazo[1,2-a]pyridine Derivatives. Russ J Gen Chem 90, 1727–1736 (2020). https://doi.org/10.1134/S1070363220090212
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070363220090212