Abstract
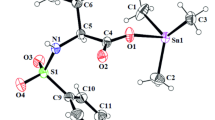
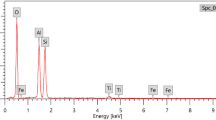
Three benzimidazole derivatives, 1-(1H-benzimidazol-2-yl)ethanol (HBE), 1H-benzimidazol-2-yl(diphenyl)methanol (BDM) and 1,2-bis(1H-benzimidazol-2-yl)ethane-1,2-diol (BHBED), have been synthesized following the one-pot rapid green protocol. Complexes of benzimidazole derivatives with six 3d transition metals, Cu(II), Mn(II), Zn(II), Fe(II), Co(II), and Ni(II), have been synthesized by free hydrothermal method. The synthesized products have been characterized by FTIR, 1H, and 13C NMR, and mass spectroscopy, and CHN analysis, and 2:1 ligand to metal stoichiometry has been confirmed. The synthesized ligands and metal complexes have been tested for antioxidant potential (DPPH), inhibitory activity including inhibition of acetylcholinesterase (AChE), butyrylcholinesterase (BChE), lipoxygenase (LOX), α-glucosidase. Micellar solubilization of the metal complexes has been studied in sodium dodecyl sulphate (SDS) by UV-Vis spectroscopy and conductivity. The selected complexes of nickel, zinc and cobalt have demonstrated interaction with SDS, and the value of critical micellar concentration increased in all cases.




Similar content being viewed by others
REFERENCES
Saify, Z.S., Kamil, A., Akhtar, S, Taha, M., Khan, A., Khan, K.M., Jahan, S., Rahim, F., Perveen, S., and Choudhary, M.I., Med. Chem. Res., 2014, vol. 23, p. 4447. https://doi.org/10.1007/s00044-014-1015-z
Shaker, S.A., Khaledi, H., Cheah, S.-C., and Ali, H.M., Arab. J. Chem., 2016, vol. 9, p. S1943. https://doi.org/10.1016/j.arabjc.2012.06.013
Liaqat, M., Mahmud, T., Hameed, A., Ashraf, M., Shafiq, M., and Asghar, H., Inorg. Nano-Met. Chem., 2017, vol. 47, p. 1418. https://doi.org/10.1080/24701556.2017.1322613
Galal, S.A., Hegab, K.H., Kassab, A.S., Rodriguez, M.L., Kerwin, S.M., Abdel-Moʼmen, A., and El Diwani, H.I., Europ. J. Med. Chem., 2009, vol. 44, p.1500. https://doi.org/10.1016/j.ejmech.2008.07.013
Wang, H.-B., and Huang, J.-M. Adv. Syn. Catal., 2016, vol. 358, p. 1975. https://doi.org/10.1002/adsc.201501167
Kovvuri, J., Nagaraju, B., Kamal, A., and Srivastava, A.K., ACS Comb. Sci., 2016, vol. 18, p. 644. https://doi.org/10.1021/acscombsci.6b00107
Zhang, Z.-H., Yin, L., and Wang, Y.-M., Cat. Comm., 2007, vol. 8, p. 1126. https://doi.org/10.1016/j.catcom.2006.10.022
Chen, C. and Chen, Y.J., Tetrahedron Lett., 2004, vol. 45, p. 113. https://doi.org/10.1016/j.tetlet.2003.10.095
Ahmad, S. Taj, M.B., Tirmizi, S. A., Alelwani, W., Hajjar, D., Makki, A., Shah, S., Ali, U., Hassan, U., and Tahir, M., Russ. J. Gen. Chem., 2019, vol. 89, p. 142. https://doi.org/10.1134/S1070363219010250
Amato, M., Caponetti, E., Martino, D.C., and Pedone, L., J. Phys. Chem. B, 2003, vol. 107, p. 10048. https://doi.org/10.1021/jp022697b
Usman, M., and Siddiq, M., Spectrochim. Acta. A, 2013, vol. 113, p. 182. https://doi.org/10.1016/j.saa.2013.04.089
Taboada, P., Ruso, J.M., Garcia, M., and Mosquera, V.C., Colloids Surf. A, 2001, vol. 179, p. 125. https://doi.org/10.1016/S0927-7757(00)00730-5
Usman, M. and Siddiq, M., J. Chem. Therm., 2013, vol. 58, p. 359. https://doi.org/10.1016/j.jct.2012.11.022
Saeed, R., Usman, M., Mansha, A., Rasool, N., Naqvi, S.A.R., Zahoor, A.F., Rahman, H.M.A., Rana, U.A., and Al-Zahrani, E., Colloids Surf. A, 2017, vol. 512, p. 51. https://doi.org/10.1016/j.colsurfa.2016.10.016
Noor, S. and Rashid, M.A., Tenside Surf. Deter., 2019, vol. 56, p. 490. https://doi.org/10.3139/113.110653
Noor, S., Younas, N., Rashid, M.A., Nazir, S., Usman, M., and Naz, T., Colloid Interface Sci. Commun., 2018, vol. 27, p. 26. https://doi.org/10.1016/j.colcom.2018.09.004
Younas, N., Rashid, M.A., Usman, M., Nazir, S., Noor, S., Basit, A., and Jamil, M., J. Surf. Det., 2017, vol. 20, p. 1311. https://doi.org/10.1007/s11743-017-1997-x
Younas, N., and Rashid, M.A., Colloid Interface Sci. Commun., 2020, vol. 35, p. 100240. https://doi.org/10.1016/j.colcom.2020.100240
Younas, N., Rashid, M.A., Nazir, S., Usman, M., Sarfraz, R.A., Jamil, A., and Whitwood, A.C., J. Mol. Liq., 2017, vol. 240, p. 351. https://doi.org/10.1016/j.molliq.2017.05.052
Taha, M., Ismail, N.H., Jamil, W., Rashwan, H., Kashif, S.M., Sain, A.A., Adenan, M.I., Ali, M., Rahim, F., and Khan, K.M., Eur. J. Med. Chem., 2014, vol. 84, p. 731. https://doi.org/10.1016/j.ejmech.2015.10.017
Ayhan-KIlcigİl, G., Kuş, C., Çoban, T., Can-Eke, B., Özbey, S., and Iscan, M., J. Enzyme Inhib. Med. Chem., 2005, vol. 20, p. 503. https://doi.org/10.1080/14756360500287086
Li, W., Li, W., Hu, Y., Xia, Y., Shen, Q., Nie, Z., Huang, Y., and Yao, S., Biosens. Bioelectron., 2013, vol. 47, p. 345. https://doi.org/10.1016/j.bios.2013.03.038
Güngördü, A., Sireci, N., Küçükbay, H., Birhanli, A., and Ozmen, M., Ecotoxicol. Environ. Saf., 2013, vol. 87, p. 23. https://doi.org/10.1016/j.ecoenv.2012.10.007
Yoon, Y.K., Ali, M.A., Wei, A.C., Choon, T.S., Khaw, K.Y., Murugaiyah, V., Osman, H., and Masand, V.H., Bioorg. Chem., 2013, vol. 49, p. 33. https://doi.org/10.1016/j.bioorg.2013.06.008
Baylac, P.R., Int. J. Aromather., 2003, vol. 13, p. 138. https://doi.org/10.1016/S0962-4562(03)00083-3
Zawawi, N.K.N.A. Taha, M., Ahmat, N., Wadood, A., Ismail, N.H., Rahim, F., Azam, S.S., and Abdullah, N., Bioorg. Chem., 2016, vol. 64, p. 29. https://doi.org/10.1016/j.bioorg.2015.11.006
Karaali, N.J., Turkish Chem. Soc., 2018, vol. 5, p. 971. https://doi.org/10.18596/jotcsa.440202
Usman, M., Cheema, M.A., Khan, A. Farooqi, Z.H., Mosquera, V., and Siddiq, M., J. Chil. Chem. Soc., 2013, vol. 58, p. 1842. https://doi.org/10.4067/S0717-97072013000300010
Akhtar, F., Hoque, M.A., and Khan, M.A., J. Chem. Thermodyn., 2008, vol. 40, p. 1082. https://doi.org/10.1016/j.jct.2008.03.001
Shah, A.M.K., Usman, M., Qureshi, R., Siddiq, M., and Shah, S.S., J. Chil. Chem. Soc., 2009, vol. 54, p. 134. https://doi.org/10.4067/S0717-97072009000200007
Dani, D., Bahadur, A., and Kuperkar, K., Colloid. Interface. Sci. Commun., 2015, vol. 25, p. 22. https://doi.org/10.1016/j.colcom.2018.06.002
Chari, M.A., Shobha, D., and Sasaki, T., Tetrahedron Lett., 2011, vol. 52, p. 5575. https://doi.org/10.1016/j.tetlet.2011.08.047
Yan, Q., Zang, H., Wu, C., Feng, J., Li, M., Zhang, M., Wang, L., and Cheng, B., J. Mol. Liq., 2015, vol. 204, p. 156. https://doi.org/10.1016/j.molliq.2015.01.016
Shedlovsky, T., J. Am. Chem. Soc., 1932, vol. 54, p. 1411. https://doi.org/10.1021/ja01343a020
Chambers, J.F., Stokes, J.M., and Stokes, R.H., J. Phys. Chem. A, 1956, vol. 60, p. 985. https://doi.org/10.1021/j150541a040
Kawamura, H., Manabe, M., Miyamoto, Y., Fujita, Y., and Tokunaga, S., J. Phys. Chem. A, 1989, vol. 93, p. 5536. https://doi.org/10.1021/j100351a042
ACKNOWLEDGMENTS
Authors gratefully acknowledge contributions from the Department of Chemistry, The Islamia University Bahawalpur and the Department of Chemistry, University of Agriculture, Faisalabad.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
No conflict of interest was declared by the authors.
Rights and permissions
About this article
Cite this article
Taj, M.B., Raheel, A., Alelwani, W. et al. A Swift One-Pot Solvent-Free Synthesis of Benzimidazole Derivatives and Their Metal Complexes: Hydrothermal Treatment, Enzymatic Inhibition, and Solubilization Studies. Russ J Gen Chem 90, 1533–1543 (2020). https://doi.org/10.1134/S107036322008023X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S107036322008023X