Abstract
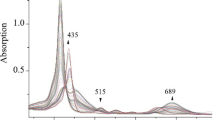
The acid-base properties of 5,10,15,20-tetraphenyl-21-oxaporphyrin and 5,10,15,20-tetraphenyl-21,23-dioxaporphyrin have been studied by means of spectrophotometric titration. The parameters of electronic absorption spectra and the concentration ranges of the existence of the obtained forms have been determined. Kinetics of the formation of complexes of 5,10,15,20-tetraphenylporphine oxa derivatives with copper at 288–308 K has been studied.




Similar content being viewed by others
REFERENCES
Berezin, B.D., Coordination Compounds of Porphyrins and Phthalocyanines, New York: John Wiley & Sons, 1981, p. 323.
Buchler, J.W., in Porphyrins, Dolphin, D., Ed., New York: Academic Press, 1978, vol. 1, p. 389.
Tarasevich, M.R. and Radyushkina, M.R., Kataliz i elektrokataliz metalloporfirinami (Catalysis and Electrocatalysis by Metal Porphyrins), Moscow: Mir, 1982, p. 168.
Senge, M.O., in The Porphyrin Handbook , Kadish, K.M., Smith, K.M., and Guilard, R., Eds., San Diego: Academic Press, 1999, vol. 1, p. 239.
da G.H. Vicente, M., and Smith, K.M., Curr. Org. Synth., 2014, vol. 11, no. 1, p. 3. https://doi.org/10.2174/15701794113106660083
Syrbu, S.A., Ageeva, T.A., Kolodina, E.A., Semeykin, A.S., and Koifman, O.I., J. Porph. Phthal., 2006, vol. 10, nos. 4–6, p. 885. https://doi.org/10.1142/S1088424606000235
Syrbu, S.A., Pukhovskaya, S.G., Nam, T.D., Ivanova, Yu.B., and Razumov, M.I., Macroheterocycles, 2019, vol. 12, no. 2, p. 135. https://doi.org/10.6060/mhc190557s
Rocheva, T.K., Shevchenko, O.G., Mazaletskaya, L.I., Sheludchenko, N.I., and Belykh, D.V., Macroheterocycles, 2018, vol. 11, no. 1, p. 95. https://doi.org/10.6060/mhc170302b
Funktsional’nye materialy na osnove tetrapirrol’nykh makrogeterotsiklicheskikh soedinenii (Functional Materials Based on Tetrapyrrole Macroheterocyclic Compounds), Koifman, O.I., Ed., Moscow: LENAND, 2019.
Ivanova, Yu.B., Pukhovskaya, S.G., Mamardashvili, N.Zh., Koifman, O.I., and Kruk, M.M., J. Mol. Liq., 2019, vol. 275, p. 491. https://doi.org/10.1016/j.molliq.2018.11.107
Latos-Grażyński, L., in The Porphyrin Handbook, Kadish, K.M., Smith, K.M., and Guilard, R., Eds., New York: Academic Press, 2000, vol. 2, p. 36.
Syrbu, S.A., Pukhovskaya, S.G., Ivanova, Yu.B., and Vashurin, A.S., Russ. J. Gen. Chem., 2019, vol. 89, no. 2, p. 255. https://doi.org/10.1134/S1070363219020142
Lisowski, J., Grzeszczuk, M., and Latos-Grażyński, L., Inorg. Chim. Acta, 1989, vol. 161, no. 153. https://doi.org/10.1016/S0020-1693(00)83086-8
Pandian, R.P. and Chandrashekar, T.K., Inorg. Chem., 1994, vol. 33, p. 3317. https://doi.org/10.1021/ic00093a020
Pandian, R.P. and Chandrashekar, T.K, J. Chem. Soc. Dalton Trans., 1993, vol. 1, p. 119. https://doi.org/10.1039/dt9930000119
Chmielewski, P.J., Latos-Grażyński, L., Olmstead, M.M., and Balch, A.L., Chem. Eur. J., 1997, vol. 3, p. 268. https://doi.org/10.1002/chem.19970030216
Chmielewski, P.J. and Latos-Grażyński, L., Inorg. Chem., 1998, vol. 37, p. 4179. https://doi.org/10.1021/ic971387i
Sridevi, B., Narayanan, S.J., Srinivasan, A., Chandrashekar, T.K., and Subramanian, J., J. Chem. Soc. Dalton Trans., 1998, vol. 12. P., 1979. https://doi.org/10.1039/a801934g
Pawlicki, M., Kańska, I., and Latos-Grażyński, L., Inorg. Chem., 2007, vol. 46, no. 16, p. 6575. https://doi.org/10.1021/ic700631t
Broadhurst, M.J., Grigg, R., and Johnson, A.W., J. Chem. Soc. (D), 1969, vol. 1480. https://doi.org/10.1039/C29690001480
Broadhurst, M.J., Grigg, R., and Johnson, A.W., J. Chem. Soc. (D), 1970, vol. 807. https://doi.org/10.1039/C29700000807
Broadhurst, M.J. and Grigg, R., J. Chem. Soc. (С), 1971, p. 3681. https://doi.org/10.1039/J3971000368.1
Latos-Grażyński, L., Lisowski, J., Olmstead, M.M., and Balch, A.L., J. Am. Chem. Soc., 1987, vol. 109, p. 4428. https://doi.org/10.1021/ja00248a067
Latos-Grażyński, L., Lisowski, J., Olmstead, M.M., and Balch, A.L., Inorg. Chem., 1989, vol. 28, no. 22, p. 4065. https://doi.org/10.1021/ic00321a005
Latos-Grażyński, L., Lisowski, J., Olmstead, M.M., and Balch, A.L., Inorg. Chem .., 1989, vol. 28, p. 1183. https://doi.org/10.1021/ic00305a032
Latos-Grażyński, L., Lisowski, J., Chmielewski, M., Grzeszczuk, M., Olmstead, M.M., and Balch, A.L., Inorg. Chem., 1994, vol. 33, p. 192. https://doi.org/10.1021/ic00080a004
Latos-Grażyński, L., Pacholska, E., Chmielewski, M., Grzeszczuk, M., Olmstead, M.M., and Balch, A.L., Inorg. Chem., 1996, vol. 35, p. 556. https://doi.org/10.1021/ic950329z
Gross, Z., Saltsman, I., Pandian, R.P., and Barzilay, C., Tetrahedron Lett., 1997, vol. 38, p. 2383. https://doi.org/10.1016/S0040-4039(97)00357-2
Andrianov, V.G. and Malkova, O.V., Macroheterocycles, 2009, vol. 2, no. 2, p. 130. https://doi.org/10.6060/mhc2009.2.130
Tagawa, K., Mori, S., Okujima, T., and Takas, M., Tetrahedron, 2017, vol. 73, p. 794. https://doi.org/10.1016/j.tet.2016.12.067
Pukhovskaya, S.G., Ivanova, Yu.B., Nam, D.T., and Vashurin, A.S., Russ. J. Phys. Chem., 2014, vol. 88, no. 10, p. 1670. https://doi.org/10.1134/S0036024414100288
Kuvshinova, E.M., Pukhovskaya, S.G., Golubchikov, O.A., and Berezin, B.D., Koord. Khim., 1993, vol. 19, no. 8, p. 630.
Gordon, A. and Ford, R., The Chemist’s Companion, New York: Wiley, 1972.
Weissberger, A., Proskauer, E., and Riddic, J., Organic Solvents, New York: Interscience Publishers, 1955
Karyakin, Yu.V. and Angelov, I.I., Chistye khimicheskie reaktivy (Pure Chemicals), Moscow: Khimiya, 1974.
Ivanova, Yu.B., Sheinin, V.B., and Mamardashvili, N.Zh., Russ. J. Gen. Chem., 2007, vol. 77, no. 8, p. 1458. https://doi.org/10.1134/S1070363207080270
Nam, D.T., Pukhovskaya, S.G., Ivanova, Y.B., Liulkovich, L.S., Semeikin, A.S., Syrbu, S.A., and Kruk, M.M., J. Incl. Phenom. Macrocycl. Chem., 2017, vol. 89, nos. 3–4, p. 325. https://doi.org/10.1007/s10847-017-0758-9
Ivanova, Yu.B., Churakhina, Yu.I., and Mamardashvili, N.Zh., Russ. J. Gen. Chem., 2008, vol. 78, no. 4, p. 673. https://doi.org/10.1134/S1070363208040269
Funding
This study was financially supported by the Russian Foundation for Basic Research (grant no. 19-03-00214 А) and performed using the equipment of the Center for Collective Usage “Upper Volga Regional Center for Physico-Chemical Investigation.”
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
No conflict of interest was declared by the authors.
Rights and permissions
About this article
Cite this article
Pukhovskaya, S.G., Ivanova, Y.B., Plotnikova, A.O. et al. Complexing and Acid-Base Properties of 5,10,15,20-Tetraphenylporphine Oxaderivatives. Russ J Gen Chem 90, 1292–1297 (2020). https://doi.org/10.1134/S1070363220070154
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070363220070154