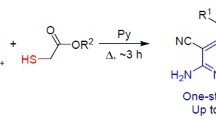
Abstract
The recyclization of 2-{[5-aryl-2-oxofuran-3(2H)-ylidene]amino}thiophene-3-carboxylic acids with cyano-acetic acid derivatives in the presence of t-BuOK afforded potassium 1-cyano-3-{[5-R1-4-R2-3-(ethoxycarbonyl)-thiophen-2-yl]amino}-1-R3-5-oxo-5-arylpenta-1,3-dien-2-olates.

Similar content being viewed by others
REFERENCES
Siutkina, A.I., Igidov, N.M., Dmitriev, M.V., Makhmudov, R.R., and Novikova, V.V., Russ. J. Gen. Chem., 2019, vol. 89, p. 1388. https://doi.org/10.1134/s1070363219070065
Shipilovskikh, S.A. and Rubtsov, A.E., Russ. J. Org. Chem., 2014, vol. 50, p. 298. https://doi.org/10.1134/s1070428014020286
Elkholy, Y.M., Ali, K.A., and Farag, A.M., J. Heterocycl. Chem., 2006, vol. 5, p. 1183. https://doi.org/10.1002/jhet.5570430508
Mayorova, O.A. and Yegorova, A.Y., Magn. Reson. Chem., 2015, vol. 10, p. 853. https://doi.org/10.1002/mrc.4270
Shipilovskikh, S.A. and Rubtsov, A.E., Russ. J. Org. Chem., 2015, vol. 50, p. 1853. https://doi.org/10.1134/s1070428014120288
Sayed, H.H., Hashem, A.I., Yousif, N.M., and ElSayed, W.A.,Arch. Pharm., 2007, vol. 6, p. 315. https://doi.org/10.1002/ardp.200700043
Maiorova, O.A., Babkina, N.V., and Egorova, A.Y., Chem. Heterocycl. Compd., 2015, vol. 51, p. 514. https://doi.org/10.1007/s10593-015-1730-5
Gavkus, D.N., Maiorova, O.A., Borisov, M.Y., and Egorova, A.Y.,Russ. J. Org. Chem., 2012, vol. 48, p. 1229. https://doi.org/10.1134/s107042801209014x
Elkholy, Y.M., Ali, K.A., and Farag, A.M., Phosphorus, Sulfur, Silicon, Relat. Elem., 2006, vol. 181, p. 2037. https://doi.org/10.1080/10426500600605731
Maiorova, O.A., Grinev, V.S., and Yegorova, A.Y., J. Struct. Chem., 2015, vol. 56, p. 803. https://doi.org/10.1134/s0022476615040320
Igidov, N.M., Zakhmatov, A., and Rubtsov, A.E., Russ. J. Org. Chem., 2016, vol. 52, p. 974. https://doi.org/10.1134/s1070428016070083
Abu El-Azm, F.S.M., Ali, A.T., and Hekal, M.H., Org. Prep. Proc. Int., 2019, vol. 51, p. 507. https://doi.org/10.1080/00304948.2019.1666635
Aly, H.M., Saleh, N.M., and Elhady, H.A., Eur. J. Med. Chem., 2011 Vol. 46, p. 4566. https://doi.org/10.1016/j.ejmech.2011.07.035
El-Borai, M.A., Rizk, H.F., Ibrahim, S.A., and Fares, A.K.,J. Heterocycl. Chem., 2019, vol. 56, p. 2787. https://doi.org/10.1002/jhet.3658
El-Malah, A., Abouelatta, A.I.Y., Mahmoud, Z., and Salem, H.H.,J. Mol. Struct. 2019, vol. 1196, p. 162. https://doi.org/10.1016/j.molstruc.2019.06.071
Fayed, A.A., Alahmadi, Y.M., Yousif, M.N.M., Yousif, N.M., Amer, A.A., El-Farargy, A.F., Ouf, N.H., and Gad, F.A., Russ. J. Gen. Chem., 2019, vol. 89, p. 1887. https://doi.org/10.1134/s1070363219090251
Fogue, P.S., Lunga, P.K., Fondjo, E.S., De Dieu Tamokou, J., Thaddee, B., Tsemeugne, J., Tchapi, A.T., and Kuiate, J.R., Mycoses, 2012, vol. 55, p. 310. https://doi.org/10.1111/j.1439-0507.2011.02089.x
Fyfe, T.J., Zarzycka, B., Lim, H.D., Kellam, B., Mistry, S.N., Katrich, V., Scammells, P.J., Lane, J.R., and Capuano, B., J. Med. Chem., 2019, vol. 62, p. 174. https://doi.org/10.1021/acs.jmedchem.7b01565
Mohamed, M.F.A., Youssif, B.G.M., Shaykoon, M.S.A., Abdelrahman, M.H., Elsadek, B.E.M., Aboraia, A.S., and Abuo-Rahma, G.E.A., Bioorg. Chem., 2019, vol. 91, p. 103127. https://doi.org/10.1016/j.bioorg.2019.103127
Puthran, D., Poojary, B., Purushotham, N., Harikrishna, N., Nayak, S.G., and Kamat, V., Heliyon, 2019, vol. 5, p. 02233. https://doi.org/10.1016/j.heliyon.2019.e02233
Bozorov, K., Nie, L.F., Zhao, J., and Aisa, H.A., Eur. J. Med. Chem., 2017, vol. 140, p. 465. https://doi.org/10.1016/j.ejmech.2017.09.039
Hawksley, D., Griffin, D.A., and Leeper, F.J., J. Chem. Soc. Perkin Trans. 1, 2001, p. 2. https://doi.org/10.1039/b006962k
Puterova, Z., Krutosikova, A., and Vegh, D., Arkivoc, 2010, vol. 1, p. 209. https://doi.org/10.3998/ark.5550190.0011.105
Igidov, N.M., Kiselev, M.A., and Rubtsov, A.E., Russ. J. Org. Chem., 2016, vol. 52, p. 526. https://doi.org/10.1134/s1070428016040084
Komarova, O.A., Igidov, N.M., Rubtsov, A.E., Zalesov, V.V., Makarov, A.S., and Toksarova, Y.S., Russ. J. Org. Chem., 2010, vol. 46, p. 236. https://doi.org/10.1134/s1070428010020156
Pulina, N.A. Kuznetsov, A.S., and Rubtsov, A.E., Russ. J. Org. Chem., 2015, vol. 51, p. 967. https://doi.org/10.1134/S1070428015070131
Rubtsov, A.E. and Zalesov, V.V., Russ. J. Org. Chem., 2003, vol. 39, p. 869. https://doi.org/10.1023/B:RUJO.0000003167.28537.71
Tyuneva, A.V., Igidov, N.M., Koryagina, N.N., Borodin, A.Y., Zakhmatov, A.V., Makarov, A.S., Toksarova, Y.C., and Rubtsov, A.E., Russ. J. Org. Chem., 2011, vol. 47, p. 258. https://doi.org/10.1134/S1070428011020163
Shipilovskikh, S.A., Makhmudov, R.R., Lupach, D.Y., Pavlov, P.T., Babushkina, E.V., and Rubtsov, A.E., Pharm. Chem. J., 2013, vol. 47, p. 366. https://doi.org/10.1007/s11094-013-0960-z
Shipilovskikh, S.A. and Rubtsov, A.E., Russ. Chem. Bull., 2015, vol. 63, p. 2205. https://doi.org/10.1007/s11172-014-0722-4
Vasileva, A.Y., Vaganov, V.Y., Shipilovskikh, S.A., and Rubtsov, A.E., Russ. J. Org. Chem., 2018, vol. 54, p. 582. https://doi.org/10.1134/s1070428018040115
Shipilovskikh, S.A., Shipilovskikh, D.A., and Rubtsov, A.E.,Russ. J. Org. Chem., 2017, vol. 53, p. 137. https://doi.org/10.1134/s1070428017010274
Shipilovskikh, S.A. and Rubtsov, A.E., J. Org. Chem., 2019,vol. 84, p. 15788. https://doi.org/10.1021/acs.joc.9b00711
Shipilovskikh, S.A., Rubtsov, A.E., and Zalesov, V.V., Chem. Heterocycl. Compd., 2009, vol. 45, p. 658. https://doi.org/10.1007/s10593-009-0334-3
Funding
This work was financially supported by the Russian Science Foundation (project no. 18-73-00091).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
No conflict of interest was declared by the authors.
Rights and permissions
About this article
Cite this article
Shipilovskikh, S.A., Rubtsov, A.E. Recyclization of 3-(Thiophen-2-yl)imino-3H-furan-2-ones under the Action of Cyanoacetic Acid Derivatives. Russ J Gen Chem 90, 809–814 (2020). https://doi.org/10.1134/S1070363220050084
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070363220050084