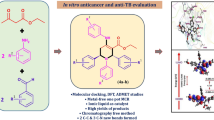
Abstract
Synthesis of a novel series of chalcone based 1,2,3-triazole derivatives and their anti-cancer studies are presented. Majority of the target compounds exhibit moderate to excellent activity against A-549 cells. Among the compounds screened 7c and 7j demonstrate potent activity being compared with the standard drug Doxorubicin.

Similar content being viewed by others
REFERENCES
Mahapatra, D. K., Asati, V., and Bharti, S.K., Eur. J. Med. Chem., 2015, vol. 92, p. 839. https://doi.org/10.1016/j.ejmech.2015.01.051
Chen, M., Christensen, S.B., Zhai, L., Rasmussen, M., Theander, T., Frøkjaer, S., Steffansen, B., Davidsen, J., and Kharazmi, A., J. Infect. Dis., 1997, vol. 176, no. 5, p. 1327. https://doi.org/10.1086/514129
Sunitha, V., Kumar, A.K., Mahesh, M., Shankaraiah, P., Jalapathi, P., and Lincoln, C.A., Russ. J. Gen. Chem., 2018, vol. 88, no. 9, p. 1904-1911. https://doi.org/10.1134/S1070363218090232
Kantevari, S., Addla, D., Bagul, P.K., Sridhar, B., and Banerjee, S.K., Bioorg. Med. Chem., 2011, vol. 19, no. 16, p. 4772. https://doi.org/10.1016/j.bmc.2011.06.085
Mishra, L., Itokawa, H., Bastow, K.F., Tachibana, Y., Nakanishi, Y., Kilgore, N., Lee, K.-H., and Sinha, R., Bioorg. Med. Chem., 2001, vol. 9, no. 7, p. 1667. https://doi.org/10.1016/S0968-0896(01)00074-8
Das, K., Bauman, J.D., Rim, A.S., Dharia, C., Clark, Jr.A.D., Camarasa, M.-J., Balzarini, J., and Arnold, E., J. Med. Chem., 2011, vol. 54, no. 8, p. 2727. https://doi.org/10.1021/jm101536x
Sunitha, V., Kumar, A.K., Shankar, B., Kumar, A.A., Krishna, T., Lincoln, C.A., and Pochampalli, J., Russ. J. Gen. Chem., 2017, vol. 87, no. 2, p. 322. https://doi.org/10.1134/S1070363217020281
Kumar, A.K., Sunitha, V., Shankar, B., Krishna, T.M., Lincoln, C.A., and Jalapathi, P., Russ. J. Gen. Chem., 2017, vol. 87, no. 9, p. 2011. https://doi.org/10.1134/S1070363217090171
Sunitha, V., Kumar, A. K., Shankaraiah, P., Jalapathi, P., and Lincoln, C.A., Russ. J. Gen. Chem., 2018, vol. 88, no. 7, p. 1515. https://doi.org/10.1134/S1070363218070265
Kumar, A.K., Sunitha, V., Shankaraiah, P., Siddhartha, M., and Jalapathi, P., Russ. J. Gen. Chem., 2018, vol. 88, no. 4, p. 789. https://doi.org/10.1134/S1070363218040254
Kumar, A.K., Sunitha, V., Shankar, B., Ramesh, M., Krishna, T.M., and Jalapathi, P., Russ. J. Gen. Chem., 2016, vol. 86, no. 5, p. 1154. https://doi.org/10.1134/S1070363216050297
Kumar, K., Pradines, B., Madamet, M., Amalvict, R., and Kumar, V., Eur. J. Med. Chem., 2014, vol. 86, p. 113. https://doi.org/10.1016/j.ejmech.2014.08.053
Patpi, S. R., Pulipati, L., Yogeeswari, P., Sriram, D., Jain, N., Sridhar, B., Murthy, R., Kalivendi, S.V. and Kantevari, S., J. Med. Chem., 2012, vol. 55, no. 8, p. 3911. https://doi.org/10.1021/jm300125e
Yadav, P., Lal, K., Kumar, A., Guru, S.K., Jaglan, S., and Bhushan, S., Eur. J. Med. Chem., 2017, vol. 126, p. 944. https://doi.org/10.1016/j.ejmech.2016.11.030
Parveen, Z., Brunhofer, G., Jabeen, I., Erker, T., Chiba, P., and Ecker, G.F., Bioorg. Med. Chem., 2014, vol. 22, no. 7, p. 2311. https://doi.org/10.1016/j.bmc.2014.02.005
Hans, R.H., Guantai, E.M., Lategan, C., Smith, P.J., Wan, B., Franzblau, S.G., Gut, J., Rosenthal, P.J., and Chibale, K., Bioorg. Med. Chem. Lett, 2010, vol. 20, no. 3, p. 942. https://doi.org/10.1016/j.bmcl.2009.12.062
Kant, R., Kumar, D., Agarwal, D., Gupta, R.D., Tilak, R., Awasthi, S.K., and Agarwal, A., Eur. J. Med. Chem., 2016, vol. 113, p. 34. https://doi.org/10.1016/j.ejmech.2016.02.041
Dalla Via, L., Gia, O., Chiarelotto, G., and Ferlin, M.G., Eur. J. Med. Chem., 2009, vol. 44, no. 7, p. 2854. https://doi.org/10.1016/j.ejmech.2008.12.011
ACKNOWLEDGMENTS
M. Raghavender thanks the Head of the Department, Osmania University, Hyderabad, India and Central Facilities for Research and Development, Osmania University, Hyderabad, India for providing analytical support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
No conflict of interest was declared by the authors.
Rights and permissions
About this article
Cite this article
Raghavender, M., Kumar, A.K., Sunitha, V. et al. Synthesis and Cytotoxicity of Chalcone Based 1,2,3-Triazole Derivatives. Russ J Gen Chem 90, 697–702 (2020). https://doi.org/10.1134/S1070363220040210
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070363220040210