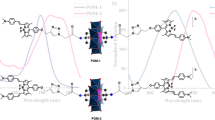
Abstract
Boron dipyrrins (Bodipy) are unique complexes occupying an intermediate position between classical coordination and organometallic compounds. The enormous interest of researchers from all over the world to the compounds of this class has by now provided an endless stream of publications that requires generalization. Existing reviews and original publications do not fully cover the structural diversity of Bodipy and their possible applications in engineering, medicine, analytical chemistry, optics, photonics, etc. In this paper, w e present the results, mainly of our own research, which for the first time allowed us to establish the physicochemical principles of the formation of Bodipy (the reasons for the low rates of complexation of ligands with boron trifluoride), changes in the spectral and photophysical properties of alkylated Bodipy complexes with the separation of contributions from universal and specific solvation, and examples of functionalization of inorganic and organic polymer materials based on Bodipy of different structures. Examples of the current team’s Bodipy research in different practical areas are presented.
Similar content being viewed by others
References
Akchurin, I.O., Bochkov, A.Yu., and Traven’, V.F., Usp. Khim. Khim. Tekhnol., 2011, vol. 25, no.11, issue 127, pp. 36–40.
Efimov, V.A., Fedyunin, S.V., and Chakhmakhcheva, O.G., Bioorg. Khim., 2011, vol. 37, no. 2, pp. 278–283.
Pakhomov, A.A., Martynov, V.I., Kononevich, Y.N., Korlyukov, A.A., and Muzafarov, A.M., Mendeleev Commun., 2016, vol. 26, no. 3, pp. 196–198.
Jiang, X.-D., Li, S., Guan, J., Fang, T., Liu, X., and Xiao, L.-J., Curr. Org. Chem., 2016, vol. 20, no. 16, pp. 1736–1744.
Boens, N., Verbelen, B., and Dehaen, W., Eur. J. Org. Chem., 2015, vol. 2015, no. 30, pp. 6577–6595.
Schmitt, A., Hinkeldey, B., Wild, M., and Jung, G., J. Fluoresc., 2009, vol. 19, no. 4, pp. 755–759.
Tram, K., Yan, H., Jenkins, H.A., Vassiliev, S., and Bruce, D., Dyes Pigments, 2009, vol. 82, no. 3, pp. 392–395.
Descalzo, A.B., Xu, H.-J., Xue, Z.-L., Hoffmann, K., Shen, Z., et al., Org. Lett., 2008, vol. 10, pp. 1581–1584.
Haugland, R.P., Handbook of Fluorescent Probes and Research Chemicals, 6th ed, Molecular Probes: Eugene. OR. 1996.
Awuah, S.G., Polreis, J., and Biradar, V., J. Org. Lett., 2011, vol. 13, pp. 3884–3887.
Boyer, J.H., Haag, A.M., Sathyamoorthi, G., Soong, M.L., Thangaraj, K., and Pavlopouloset, T.G., Heteroat. Chem., 1993, vol. 4, pp. 39–49.
Yogo, T., Urano, Y., Mizushima, A., Sunahara, H., Inoue, T., Hirose, K., Iino, M., Kikuchi, K., and Nagano, T., PNAS, 2008, vol. 105, no. 1, pp. 28–32.
Pang, W., Zhang, X.-F., Zhou, J., Yu, C., Hao, E., and Jiao, L., Chem. Commun., 2012, vol. 48, pp. 5437–5439.
Ran, S., Xu, X., Scott, B.R., Ferrara, B.J., Neal, K., Bacskai, B.J., Medarova, Z., and Moore, A., J. Am. Chem. Soc., 2009, vol. 131, pp. 15257–15261.
Yutanova, S.L., Kuznetsova, R.T., Aksenova, Yu.V., Tel’-minov, Ye.N., and Berezin, M.B., Izv. Vyssh. Uchebn. Zaved. Fiz., 2013, vol. 56, no. 3, pp. 23–27.
Driessen, O. and Lugtenberg, J., J. High Resolution Chromatogr. Chromatogr. Commun., 1980, vol. 3, pp. 405.
Goze, C., Ulrich, G., Charbonniere, L., Cesario, M., and Prange, T., Chem. Eur. J., 2003, vol. 9, pp. 3748–3752.
Coskun, A., Baytekin, B.T., and Akkaya, E.U., Tetrahedron Lett., 2003, vol. 44, pp. 5649–5653.
Cha, N.R., Moon, S.Y., and Chang, S.-K., Tetrahedron Lett., 2003, vol. 44, pp. 8265–8270.
Kollmannsberger, M., Rurack, K., Resch-Genger, U., Rettig, W., and Daub, J., Chem. Phys. Lett., 2000, vol. 329, pp. 363–367.
Rurack, K., Sczepan, M., Spieles, M., Resch-Genger, U., and Rettig, W., Chem. Phys. Lett., 2000, vol. 320, pp. 87–95.
Gabe, Y., Urano, Y., Kikuchi, K., Kojima, H., and Nagano, T., J. Am. Chem. Soc., 2004, vol. 126, pp. 3357–3363.
Rurack, K., Kollmannsberger, M., and Daub, J., New J. Chem., 2001, vol. 25, pp. 289–295.
Gareis, T., Huber, C., and Wolfbeis, O.S., J. Chem. Commun., 1997, pp. 1717–1719.
Baki, C.N. and Akkaya, E.U., J. Org. Chem., 2001, vol. 66, pp. 1512–1513.
Soper, S.A., Owens, C., Lassiter, S., Xu, Y., and Waddell, E., Topics in Fluorescence Spectroscopy, Lakowicz, J.R., Ed., New York: Kluwer, 2003, vol. 7, pp. 1–68.
Ulrich, G., Goze, C., Guardigli, M., Roda, A., and Ziessel, R., Ang. Chem. Int. Ed., 2005, vol. 44, pp. 3694–3698.
Levitt, J.A., Kuimova, M.K., Yahioglu, G., Chung, P., and Suhling, K., J. Phys. Chem. C., 2009, vol. 113, pp. 11634–11642.
Erten-Ela, S., Yilmaz, M.D., Icli, B., Dede, Y., Icli, S., and Akkaya, E.U., Org. Lett., 2008, vol. 10, pp. 3299–3302.
Sabatini R.P., McCormick T., Lazarides T., Wilson K.C., Eisenberg, R., and McCamant, D. W., J. Phys. Chem. Lett., 2011, no. 3, pp. 223–227. doi: 10.1021/jz101697y
Sazanovich, I.V., Kirmaier, Ch., Hindin, E., Yu, L., Bocian, D.F., Lindsey, J.S., and Holten, D., J. Am. Chem. Soc., 2004, vol. 126, pp. 2664.
Surya, P. and Gayathri, T., EurJOC, 2014, vol. 2014, no. 22, pp. 4689–4707.
Yang, L., Liu, J.H., Liang, H., and Jianrong, G., J. Photo-chem. Photobiol. A: Chem., 2017, vol. 332, pp. 283.
Yamazawa, S., Nakashima, M., Suda, Y., Nishiiabu, R., and Kubo, Y., J. Org. Chem., 2016, vol. 81, no. 3, pp. 1310–1315.
Yakubovskyi, V., Didukh, N., Zatsikha, Yu., and Kovtun, Yu., Chem. Select, 2016, vol. 1, no. 7, p. 1462
Pang, W., Zhang, X.-F., Zhou, J., Yu, C., and Hao, E., Chemical communications (Cambridge, England). 2012, vol. 48, pp. 5437–5439.
Rumyantsev, E.V., Aleshin, S.N., Desoki, A., Marfin, Yu.S., and Antina, E.V., Russ. J. InOrg. Chem., 2013, vol. 58, no. 5, p. 506. 10.1134/S0036023613050197
Rumyantsev, E.V., Desoki, A., Marfin, Yu.S., and Antina, E.V., Russ. J. Gen. Chem., 2010, vol. 80, no. 9, p. 1871. https://doi.org/10.1134/S1070363210090264
Rumyantsev, E.V., Marfin, Yu.S., and Antina E.V., Izv. Akad. Nauk, Ser. Khim., 2010, no. 10, pp. 1840–1845.
Gonzalez-Valls, Mirloup, A., Bahers, T.Le, Keller, N., Cottineau, T., Sautet, P., and Keller, V., RSC Adv., 2016, vol. 6, no. 94, pp. 91529–91540.
Bol’shakov, G.F., Infrakrasnye spektry i rentgeno-grammy geteroorganicheskikh soedinenii (Infrared Spactra and X-Day Patterns of Hetero-Organic Compounds), Leningrad: Khimiia, 1967.
Goze, C., Ulrich, G., and Ziessel, R., Org. Lett., 2006, vol. 8, no. 20, pp. 4445–4448.
Gur’yanova, E.N., Donorno-aktseptornaya svyaz’ (Donor-Acceptor Bond), Moscow: Khimiia, 1973.
Andrews, L.J. and Keefer, R.M., Molecular Complexes in Organic Chemistry, San Francisco: Holden-Day, 1964.
Graham, W.A.G. and Stone, F.G.A., J. Inorg. Nucl. Chem., 1956, vol. 3, pp. 165–177.
Volkov, A.F., Romm, I.P., Gur’yanova, Ye.N., and Kocheshkov, K.A., Izv. Akad. Nauk SSSR, Ser. Khim., 1976, no. 6, pp. 1365–1367.
Grabowski, S., Hydrogen Bonding - New Insights, Dordrecht: Springer, 2006. 519 p.
Shmidt, F.K., Koordinatsionno-khimicheskie osnovy metal-lokompleksnogo kataliza (Coordination Chemical Principles of Metallocomplex Catalysis), Irkutsk: Irkutsk. Gos, Univ., 1981.
Marfin, Yu.S., Rumyantsev, E.V., Fadeev, Ya.S., and Antina, E.V., Russ J. Phys. Chem. A, 2012, vol. 86, no. 7, p. 1068. https://doi.org/10.1134/S0036024412070163
Aleksakhina, E.L., Marfin, Yu.S., Merkushev, D.A., Tomilova, I.K., and Rumyantsev, E.V., Kazan. Med. Zh., 2015, vol. 96, no. 5, pp. 792–798.
Marfin, Yu.S., Merkushev, D.A., Rumyantsev, E.V., Aleksakhina, E.L., and Tomilova, I.K., J. Fluoresc., 2016, vol. 26, no. 1, pp. 255–261.
Marfin, Yu.S., Shipalova, M.V., Kurzin, V.O., Ksenofon-tova, K.V., Solomonov, A.V., and Rumyantsev, E.V., J. Fluoresc., 2016, vol. 26, no. 6, pp. 2105–2112.
Marfin, Yu.S., Vodyanova, O.S., Merkushev, D.A., Usoltsev, S.D., Kurzin, V.O., and Rumyantsev, E.V., J. Fluoresc., 2016, vol. 26, no. 6, pp. 1975–1985.
Bobrov, A.V., Marfin, Yu.S., Kuznetsov, V.V., and Rumyantsev, E.V., Mater. Technol., 2016, pp. 1–8. https://doi.org/10.1080/10667857.2016.1154261.
Duman, S., Cakmak, Y., Kolemen, S., Akkaya, E., and Dede, Y., Org. Chem., 2012, vol. 77, pp. 4516–4527
Buyuktemiz, M., Duman, S., and Dede, Y., J. Phys. Chem. A, 2013, vol. 117, pp. 1665–1669.
Antina, E.V., Khimiya bilirubina i еgo analogov (Chemistry of Bilirubin and Its Analogs), Moscow: KRASAND, 2009.
Pardoen, J.A., Lugtenburg, J., and Canters, G.W., J. Phys. Chem., 1985, vol. 89, pp. 4272–4277.
Lee, J-S., Kim, H.K., Feng, S., Vendrell, M., and Chang, Y.-T., Chem. Commun., 2011, vol. 47, pp. 2339–2341.
López Arbeloa, F., Bañuelos, J., Martínez, V., Arbeloa, T., and López Arbeloa, I., Int. Rev. Phys. Chem., 2005, vol. 24, pp. 339–374.
Werner, T., Huber, C., Heinl, S., Kollmannsberger, M., Daub, J., and Wolfbeis, O.S., Fresenius J. Anal. Chem., 1997, vol. 359, pp. 150–154.
Thoresen, L.H., Kim, H., Welch, M.B., Burghart, A., and Burgess, K., ChemInfo, 1998, vol. 30, pp. 1276–1281.
Burghart, A., Kim, H., Welch, M.B., Thoresen, L.H., Reibenspies, J., Burgess, K., Bergström, F., and Johansson, L.B.-A., J. Org. Chem., 1999, vol. 64, pp. 7813–7819.
Rurack, K. and Kollmannsberger, M., New J. Chem., 2001, vol. 25, pp. 289–292.
Coskun, A. and Akkaya, E.U., Tetrahedron Lett., 2004, vol. 45, pp. 4947–4949.
Kang, H.C. and Haugland, R.P., US Patent 5. 433. 896, 1995.
Qin, W., Rohand, T., Baruah, M., and Stefan, A., Chem. Phys. Lett., 2006, vol. 420, pp. 562–568.
Rumyantsev, E.V., Marfin, Yu.S., Solomonov, A.V., and Timin, A.S., XX Mendeleevskii s”yezd po obshchei i prikladnoi khimii (XX Mendeleev Congress on General and Applied Chemistry), Yekaterinburg, 2016, vol. 1, p. 96.
Menges, N., Comput. Theor. Chem., 2015, vol. 1068, pp. 117–122.
Uzhinov, B.M., Ivanov, V.L., and Mel’nikov, M.Ya., Usp. Khim., 2011, vol. 80, no. 12, pp. 1231–1242.
Hattori, S., Ohkubo, K., Urano, Y., Sunahara, H., Nagano, T., Wada, Yu., Tkachenko, N.V., Lemmetyinen, H., and Fukuzumi, S., J. Phys. Chem. B, 2005, vol. 2005, no. 109, pp. 32–15368.
Hu, R., Lager, E., Aguilar-Aguilar, A., Liu, J., Lam, J.W.Y., Sung, H.H.Y., Williams, I.D., Zhong, Y., Wong, K.S., Peña-Cabrera, E., and Tang, B.Z., J. Phys. Chem. C, 2009, vol. 113, no. 36, pp. 15845–15853.
Scalise, R.E., Caradonna, P.A., Tracy, H.J., Mullin, J.L., and Keirstead, A.E., J. Inorg. Organomet. Polym. Mater., 2014, vol. 24, no. 2, pp. 431–441.
Rumyantsev, E.V., Aleshin, S.N., and Marfin, Yu.S., Russ. J. Phys. Chem. A, 2013, vol. 87, no. 2, p. 326–323. https://doi.org/10.1134/S003602441302026X
Marfin, Yu.S., Merkushev, D.A., Levshanov, G.A., and Rumyantsev, E.V., J. Fluoresc, 2014, vol. 24, no. 6, pp. 1613–1619.
Usol’tsev, S.D., Marfin, Yu.S., and Rumyantsev, E.V, X Konkurs proektov molodykh uchenykh v ramkakh vystavki “Khimiya, khimicheskaya promyshlennost’ i nauka” (X Competition of projects of young scientists in the framework of the exhibition “Chemistry, Chemical Industry, and Science”), Moscow, 2016, pp. 26–27.
Kazak, A., Marfin, Yu, Usoltsev, S., Smirnova, A., Rumyantsev, E., Soldatenko, E., Chumakov, A., Glukhovskoi, E., and Usoltseva, N., Abstract Book AEM2016, Univ. Surrey, 2016, p. 42.
Vodyanova, O.S., Kochergin, B.A., Marfin, Yu.S., and Rumyantsev, E.V, Abstracts of Papers, XIII Mezhdunarodnaya konferentsiya “Spektroskopiya koordinatsionnykh soedinenii” (XIII Int. Conf. “Spectroscopy of Coordination Compounds”), pos. Shepsi, Kuban. Gos. Univ., 2016, pp. 177–178.
Marfin, Yu.S., Rumyantsev, E.V., Usol’tsev, S.D., Molchanov, EYe., Kurzin, VO. et al., Abstracts of Papers, Klaster konferentsii po organicheskoi khimii “OrgKhim-2016” (Cluster Conf. on Organic Chemistry “OrgChem-2016”), St. Petersburg, 2016, p. 389.
Sunahara, H., Urano, Y., Kojima, H., and Nagano, T., J. Am. Chem. Soc, 2007, vol. 129, no. 17, pp. 5597–5604.
Qin, W., Rohand, T., Baruah, M., and Stefan, A., Chem. Phys. Lett., 2006, vol. 420, pp. 562–568.
Practical Protein Chemistry: A Handbook, Darbre, A. and Waterfield, M.D., Eds., Chichester: Wiley-Blackwell, 1986.
Panteleev, M.A. and Ataullakhanov, F.I., Klin. Onkogematol. Fund. Issled. Klin. Prakt., 2008, vol. 1, pp. 174–181.
Butenas, S. and Mann, K.G, Biokhimiya, 2002, vol. 67, no. 1, pp. 5–15.
Aleksakhina, E.L., Marfin, Yu.S., Merkushev, D.A., Tomilova, I.K., and Rumyantsev, E.V., Kazan. Med. Zh., 2015, vol. 96, no. 5, pp. 792–798.
Bobrov, A.V., Marfin, Yu.S., Kuznetsov, V.V., and Rumyantsev, E.V, Mater. Technol., 2016, pp. 1–8. https://doi.org/10.1080/10667857.2016.1154261.
Bobrov, A.V., Marfin, Yu.S., and Rumyantsev, E.V., Abstracts of Papers, Mezhdunarodnyi molodezhnyi nauchnyi forum “LOMONOSOV-2015” (Int. Youth Scientific Forum “LOMONOSOV-2015”, Moscow, 2015, p. 167.
Merkushev, D.A., Marfin, Yu.S., and Rumyantsev, E.V., Abstracts of papers, XIV Konferentsiia molodykh uchenykh “Aktual’ nye problemy neorganicheskoi khimii” (XIV Conf. of Young Scientists “Actual Problems of Inorganic Chemistry”), Zvenigorod, 2015, p. 164.
Merkushev, D.A., Pushkarev, A.P., Marfin, Yu.S., and Rumyantsev, E.V., Abstracts of Papers, X Vserossiiskaya shkola-konferentsiya molodykh uchenykh “Teoretiches-kaya i eksperimental’ naya khimiya zhidkofaznykh sistem” (Krestovskie chteniya) [X All-Russian School-Conference of Young Scientists “Theoretical and Experimental Chemistry of Liquid-Phase Systems” (Krestov Radings)], Ivanovo, 2015, p. 186.
Merkushev, D.A., Bobrov, A.V., Marfin, Yu.S., and Rumyantsev, E.V., Abstracts of Papers, Mezhdunarodnaya nauchno-tekhnicheskaya konferentsiya “Polimernyye kompozity i tribologiia (Plikomtrib-2015) [Int. Scientific and Technical Conf. “Polymer Composites and Tribology (Pliktribrib-2015)”, Gomel, 2015, p. 54.
Shipalova, M.V., Marfin, Yu.S., and Rumyantsev, E.V., Abstracts of Papers, X Vserossiiskaya shkola-konferen-tsiya molodykh uchenykh “Teoreticheskaya i eksperi-mental’ naya khimiya zhidkofaznykh sistem” (Krestovskie chteniya) [X All-Russian School-Conference of Young Scientists “Theoretical and Experimental Chemistry of Liquid-Phase Systems” (Krestov Radings)], Ivanovo, 2015, p. 68.
Rumyantsev, E.V. and Marfin, Yu.S., Abstracts of Papers, XII Mezhdunarodnaya konferentsiya “Spektroskopiya koordinatsionnykh soedinenii” (XII Int. Conf. “Spe-ktroscopy of Coordination Compounds”, Tuapse, 2015, pp. 86–87.
Funding
The work was financially supported in part by the Grant of the President of the Russian Federation for support of young Russian candidates of sciences (MK-8835.2016.3) and grants of the Russian Foundation for Basic Research (project nos. 15-33-20002, 15-43-03214, 16-03-01028) and performed using resources of the Center for Collective Use, Ivanovo State University of Chemical Technology.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
No conflict of interest was declared by the authors.
Additional information
Russian Text © The Author(s), 2017, published in Rossiiskii Khimicheskii Zhurnal, 2017, Vol. 61, No. 3, pp. 143–162.
Rights and permissions
About this article
Cite this article
Rumyantsev, E.V., Marfin, Y.S. Boron Dipirrins: Mechanism of Formation, Spectral and Photophysical Properties, and Directions of Functionalization. Russ J Gen Chem 89, 2682–2699 (2019). https://doi.org/10.1134/S1070363219120454
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070363219120454