Abstract
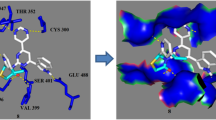
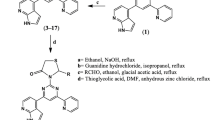
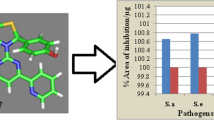
The designed molecular structures have been subjected to computational analysis for calculating their physicochemical properties and drug likeness. The calculated data indicate that most of the compound possess the bioactivity score in the active zone. Synthetic approach to the target compounds is straightforward and easy to handle. Structures of the new compounds are supported by FT-IR, 1H, and 13C NMR, and mass spectra. Antimicrobial tests of the products against pathogens (S. aureus, S. epidermidis, E. coli, and P. mirabilis) indicate the products as active or highly active. Their cyto-toxicity is determined to be 92–98% at concentration of 3.125 µmol/L. The molecular docking analysis carried out for the target compounds against the receptor Glc-N-6P exhibits low binding energy and various binding sites of those.
Similar content being viewed by others
References
Myznikov, L.V., Hrabalek, A., and Koldobskii, G.I., Chem. Heterocycl. Comp., 2007, vol. 43, p. 1. https://doi.org/10.1007/s10593-007-0001-5
Soliman, H.A., Kalmouch, A., Awad, H.M., and Abdel Wahed, N.A.M., Russ. J. Gen. Chem., 2018, vol. 88, p. 1726. https://doi.org/10.1134/S1070363218080273
Issell, B.F., Cancer Chemother. Pharmacol., 1982, vol. 7, p. 73. https://doi.org/10.1007/BF00254525
Arshad, M., Bhat, A.R., Pokharel, S., Lee, E.J., Athar, F., and Choi, I., Eur. J. Med. Chem., 2014, vol. 71, p. 229. https://doi.org/10.1016/j.ejmech.2013.11.008
Marc, A.I., Bernard, M., Stephanie, R., Andrea, S., Gheorghe, C., Valentin, C., and Claudiu, T.S., Bioorg. Med. Chem., 2004, vol. 12, p. 2717. https://doi.org/10.1016/j.bmc.2004.03.008
Bhat, A.R., Arshad, M., Lee, E.J., Pokharel, S., Choi, I., and Athar, F., Chem. Biod., 2013, vol. 10, p. 2267. https://doi.org/10.1002/cbdv.201300009
Molinspiration Cheminformatics. Nova ulica, SK-90026 Slovensky Grob, Slovak Republic. [Online] Available from: http://www.molinspiration.com [Accessed on 3rd July, 2012].
Verma, A., Asian Pac. J. Trop. Biomed., 2012, vol. 2, p. S1735. https://doi.org/10.1016/S2221-1691(12)60486-9.
Alodeani, E.A., Arshad, M., and Izhari, M.A., Eur. J. Pharm. Med. Res., 2017, vol. 4, p. 447. https://www.ejpmr.com/admin/assets/article_issue/1490961035.pdf.
Alodeani, E.A., Arshad, M., and Izhari, M.A., Eur. J. Pharm. Med. Res., 2015, vol. 2, p. 296. http://www.ejpmr.com/admin/assets/article_issue/1446625932.pdf
Arshad, M., Russ J Gen Chem., 2018, vol. 88, p. 1886. https://doi.org/10.1134/S1070363218090207
Alodeani, E.A., Arshad, M., and Izhari, M.A., Asian Pac. J. Trop Biomed., 2015, vol. 5, p. 676. https://doi.org/10.1016/j.apjtb.2015.04.010
Arshad, M., Bhat, A.R., Hoi, K.K., Choi, I., and Athar, F., Chin. Chem. Lett., 2017, vol. 28 p. 1559. https://doi.org/10.1016/j.cclet.2016.12.037
Kareem, A., Laxmi, Arshad, M., and Nishat, N., J. Photochem. Photobiol. B, 2016, vol. 160, p. 163. https://doi.org/10.1016/j.jphotobiol.2016.03.030
Iram, N., Khan, M.S., Jolly, R., Arshad, M., Alam, M., Alam, P., Khan, R.H., and Firdaus, F., J. Photochem. Photobiol. B, 2015, vol. 153, p. 20. https://doi.org/10.1016/j.jphotobiol.2015.09.001
Nami, S.A.A., Arshad, M., Shakir, M., Khan, M.S., Alam, M., Lee, D.U., Park, S., and Sarikavakli, N., Polym. Adv. Technol., 2015, vol. 26, p. 1627. https://doi.org/10.1002/pat.3591
Bushra, R., Shahadat, M., Khan, M.A., Adnan, R., Arshad, M., Rafatullah, M., and Naushad, M., Int. J. Env. Sci. Technol., 2015, vol. 12, p. 3635. https://doi.org/10.1007/s13762-014-0726-5
Nami, S.A.A., Khan, M.S., Arshad, M., Raza, M.A., and Khan, I., Polym. Adv. Technol., 2017, vol. 28, p. 10. https://doi.org/10.1002/pat.3846
Nayab, P.S., Arif, R., Arshad, M., and Rahisuddin, Heterocyc. Lett., 2015, vol. 5, p. 223. http://www.heteroletters.org/issue25/PDF/Paper-9.pdf.
Gupta, M.K., Neelakantan, TV., Sanghamitra, M., Tyagi, R.K., Dinda, A., Maulik, S., Mukhopadhyay, C.K., and Goswami, S.K., Antioxid. Redox Signal, 2006, vol. 8, p. 1081. https://doi.org/10.1089/ars.2006.8.1081
Mosmann, T., J. Immunol. Methods, 1983, vol. 65, p. 55. https://doi.org/10.1016/0022-1759(83)90303-4.
Morris, G.M., Goodsell, D.S., Halliday, R.S., Huey, R., Hart, W.E., Belew, R.K., Olson, A.J., J. Comput. Chem., 1998, vol. 19, p. 1639. https://doi.org/10.1002/(SICI)1096-987X(19981115)19:14%3C1639::AID-JCC10%3E3.0.CO;2-B.
Mouilleron, S., Badet-Denisot, M.A., and Golinelli-Pimpaneau, B., J. Mol. Biol., 2008, vol. 377, no. 4, p. 1174. https://doi.org/10.1016/j.jmb.2008.01.077
Trott, O. and Olson, A.J., J. Comput. Chem., 2010, vol. 31, p. 455. https://doi.org/10.1002/jcc.21334
Acknowledgments
The author Dr. Mohammad Arshad is highly thankful to Dr. Feras AlMarshad, the Dean College of Medicine Al-Dawadmi, Shaqra University Kingdom of Saudi Arabia for his kind cooperation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
No conflict of interest was declared by the authors.
Rights and permissions
About this article
Cite this article
Arshad, M., Khan, M.S. & Nami, S.A.A. Synthesis, Biological Activity, and Molecular Docking Assessment of Some New Sulfonylated Tetrazole Derivatives. Russ J Gen Chem 89, 1851–1858 (2019). https://doi.org/10.1134/S1070363219090202
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070363219090202