Abstract
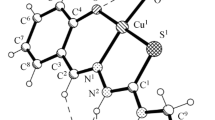
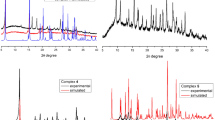
A series of new copper(I) and mercury(II) complexes with 4-aminoantipyrine, semicarbazide, and thiosemicarbazide ligands [(1,5-dimethyl-3-oxo-2-phenyl-2,3-dihydro-1H-pyrazol-4-yl)carbonohydrazonoyl dicyanide (HL1), 2-cyano-N-(1,5-dimethyl-3-oxo-2-phenyl-2,3-dihydro-1H-pyrazol-4-yl)-2-[(E)-(3-methylphenyl)-diazenyl]acetamide (H2L2), (Z)-2-cyano-N′-[(E)-(1,5-dimethyl-3-oxo-2-phenyl-2,3-dihydro-1H-pyrazol-4-yl)-methylidene]-2-(2-phenylhydrazinylidene)acetohydrazide (HL3), 2-(anilinoacetyl)-N-phenylhydrazine-1-carbothioamide (H2L4), 2-(anilinoacetyl)-N-(3-methylphenyl)hydrazine-1-carbothioamide (H2L5), and 2-anilino-N′-[(E)-(2-hydroxyphenyl)methylidene]acetohydrazide (H2L6)] have been prepared and characterized by physical and spectral data, including microanalysis, IR and UV-visible spectra, conductivity measurements, and thermal analyses (DTG/TGA). The ligands H2L2 and HL3 produced dinuclear complexes. Thermal studies revealed that the copper(I) complexes are thermally more stable than mercury(II) complexes. The copper complexes exhibited potent inhibitory effect on the MCF7 human breast carcinoma cell line, as compared to mercury(II) complexes.
Similar content being viewed by others
References
Acheson, R., An Introduction to the Chemistry of Heterocyclic Compounds, New York: Wiley, 1976, 3rd ed. p. 354.
Bansal Raj, K., Heterocyclic Chemistry, New Delhi: New Age International Publisher, 2010, 4th ed., p. 454.
Raman, N., Dhaveethuraja, J., and Sakthivel, A., J. Chem. Sci., 2007, vol. 119, p. 303. doi 10.1007/s12039-007-0041-5
Raman, N., Sakthivel, A., and Rajasekaran, K., Mycobiology, 2007, vol. 35, p. 150. doi 10.4489/MYCO.2007.35.3.150
Anupama, M., Padmaja, C., and Gyana, K., E-J. Chem., 2012, vol. 9, p. 389. doi org/10.1155/2012/291850
Zahid, H., Mohammed, S., Hummara, S., Andra, S., Claudiu, T., Enzyme Inhib. Med. Chem., 2002, vol. 17, p. 87. doi org/10.1080/14756360290030734
Selvakumar, P.M., Suresh, E., and Subramanian, P.S., Polyhedron, 2007,vol. 26, p. 749. doi org/10.1016/j.poly.2006.09.004
Zahid, H., Humayna, P., Abdul, R., Andra, S., and Claudiu, T., Enzyme Inhib. Med. Chem., 2002, vol. 17, p. 117. doi org/10.1080/14756360290024218
Singh, V.P., Gupta, P., J. Enzyme Inhib. Med. Chem., 2008, vol. 23, p. 797. doi 10.1080/14756360701733136
Guzel, O., Karali, N., and Salman, A., Bioorg. Med. Chem., 2008, vol. 16, p. 8976. doi org/10.1016/j.bmc.2008.08.050
Cocco, M., Congiu, C., Onnis, V., Pellerano, M., and De Logu, A., Bioorg. Med. Chem., 2002, vol. 10, p. 501. doi org/10.1016/S0968-0896(01)00310-8
Al-Soud, Y., Al-Dweri, M., and Al-Masoudi, N., II Farmaco, 2004, vol. 59, p. 775. doi org/10.1016/j.farmac.2004.05.006
Collins, F., Klayman, D., and Morrison, N., J. Gen. Microbiol., 1982, vol. 128, p. 1349. doi 10.1099/00221287-128-6-1349
Manoj, E. and Kurup Prathapachandra, R., Polyhedron, 2008, vol. 27, p. 275. doi org/10.1016/j.poly.2007.09.023
Murugkar, A., Unnikrishnan, B., Padhye, S., Bhonde, R., Teat, S., Triantafillou, E., and Sinn, E., Met.-Based Drugs, 1999, vol. 6, p. 177. doi org/10.1155/MBD.1999.177
Marzano, C., Pellei, M., Tisato, F., and Santini, C., Anti-Cancer Agents Med. Chem., 2009, vol. 9, p. 185. doi org/10.2174/187152009787313837
Despaigne, A.A.R., Vieira, L.F., Mendes, I.C., Da Costa, F.B., Speziali, N.L., and Beraldo, H., J. Braz. Chem. Soc., 2010, vol. 21, p. 1247. doi org/10.1590/S0103-50532010000700012
Shit, S., Dey, S.K., Rizzoli, C., Zangrando, E., Pilet, G., Gomez-Garcia, C.J., and Mitra, S., Inorg. Chim. Acta, 2011, vol. 370, p. 18. doi org/10.1016/j.ica.2011.01.008
Iskander, M.F., El-Sayed, L., Salem, N.M. H., Haase, W., Linder, H.J., and Foro, S., Polyhedron, 2004, vol. 23, p. 23. doi org/10.1016/j.poly.2003.09.022
Angelusiu, M.V., Barbuceanu, S.F., Draghici, C., and Almajan, G.L., Eur. J. Med. Chem., 2010, vol. 45, p. 2055. doi org/10.1016/j.ejmech.2010.01.033
Bakir, M., Hassan, I., Johnson, T., Brown, O., Green, O., Gyles, C., and Coley, M.D., J. Mol. Struct., 2004, vol. 688, p. 213. doi org/10.1016/j.molstruc.2003.10.017
Bikas, R., Monfared, H., Lis, T., and Siczek, M., Inorg. Chem. Commun., 2012, vol. 15, p. 151. doi org/10.1016/j.inoche.2011.10.012
El-Asmy, A.A., El-Gammal, O.A., Radwan, H.A., and Ghazy, S.E., Spectrochim. Acta, Part A, 2010, vol. 77, p. 297. doi org/10.1016/j.saa.2010.05.026
Prakash, A. and Sangamesh, P., J. Coord. Chem., 2008, vol. 61, p. 2570. doi org/10.1080/00958970801948064
El-Boraeya, H., Abdel-Rahmanb, R., Atiaa, E., and Hilmya, K., Cent. Eur. J. Chem., 2010, vol. 8, p. 820. doi 10.2478/s11532-010-0046-7
Kashar, T., Eur. Sci. J., 2013, vol. 9, p. 449. doi org/index.php/esj/article/viewFile/2121/2034
Abou Sekkina, M.M., Kashar, T.I., and Aly, S.A., Solid State Sci., 2011, vol. 13, p. 2080. doi org/10.1016/j.solidstatesciences.2011.07.015
Aly, S., J. Rad. Res. Appl. Sci., 2017, vol. 10, p. 86. doi org/10.1016/j.jrras.2016.12.001
Abou El-Enein, S.A., El-Saied, F.A., Kasher, T.I., and El-Wardany, A.H., Spectrochim. Acta, Part A, 2007, vol. 67, p. 737. doi org/10.1016/j.saa.2006.07.052
Sathisha, M.P., Revankar, V.K., and Pai, K.S.R., Met.-Based Drugs, 2008, vol. 2008, p. 1155. https://www.ncbi.nlm.nih.gov.
Gudasi, K.B., Shenoy, R.V., Vadavi, R.S., Pattil, M.S., and Patil, S.A., Chem. Pharm. Bull., 2005, vol. 53, p. 1077. doi org/10.1248/cpb.53.1077
Abd El-Wahab, Z.H., Spectrochim. Acta, Part A, 2007, vol. 67, p. 25. doi org/10.1016/j.saa.2006.05.038
Mosoarca, E.M., Tudose, R., Alexandrova, R., and Costisor, O., Chem. Bull., 2005, vol. 50, p. 52. doi org/10.1080/00958972.2010.539683
Suman, M., Suparna, G., Bhart, J., Archana, S., Mamta, B., Int. J. Inorg. Chem., 2013 vol. 2013, ArticleID549805, p. 6. doi org/10.1155/2013/549805.
Raman, N., Esthar, S., and Thangaraja, C.A., J. Chem. Sci., 2004, vol. 116, p. 209. doi 10.1007/BF02708269
El-Boraey, H.A., Abd-ElRahman, R.M., Atia, E.M., and Hilmy, K.M., Cent. Eur. J. Chem., 2010, vol. 8, p. 820. doi org/10.2478/s11532-010-0046-7
El-Boraey, H.A., El-Saied, F.A. and Aly, S.A., J. Therm. Anal. Calorim., 2009, vol. 96, p. 599. doi 10.1007/s10973-008-9197-6
El-Boraey, H.A., Emam, S.M., Tolan, D.A., and El-Nahas, A.M., Spectrochim. Acta, Part A, 2011, vol. 78, p. 360. doi org/10.1016/j.saa.2010.10.021
Sahar, M. and Magdy, M., Chem. Pharm. Bull., 2000, vol. 48, p. 266. http://cpb.pharm.or.jp/cpb/200002/C02_0266.pdf.
Skehan, P., Storeng, R., Scudiero, D., Monks, A., McMahon, J., Vistica, D., Warren, J.T., Bokesch, H., Kenney, S., and Boyd, M.R., J. Nat. Cancer Inst., 1990, vol. 82, p. 1107. doi org/10.1093/jnci/82.13.1107
Afrasiabi, Z., Sinn, E., Lin, W., Ma, Y., Campana, C., and Padhye, S., J. Inorg. Biochem., 2005, vol. 99, p. 1526. doi org/10.1016/j.jinorgbio.2005.04.012
Author information
Authors and Affiliations
Corresponding author
Additional information
The text was submitted by the author in English.
Rights and permissions
About this article
Cite this article
Aly, S.A. Synthesis, characterization, and antitumor activity of new copper(I) and mercury(II) complexes. Russ J Gen Chem 87, 1256–1263 (2017). https://doi.org/10.1134/S1070363217060214
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070363217060214