Abstract
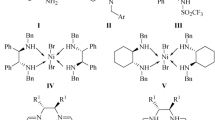
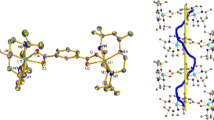
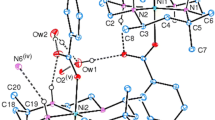
Chiral ligands—derivatives of (1R,2R)-cyclohexane-1,2-diamine, (1R,2R)-diphenylethane-1,2-diamine, and (2S,3S)-bicyclo[2.2.2]octane-2,3-diamine—and octahedral Ni(II) complexes on their basis have been synthesized.
Similar content being viewed by others
References
Suga, H., Furihata, Y., Sakamoto, A., Itoh, K., Okumura, Y., Tsuchida, T., Kakehi, A., and Baba, T., J. Org. Chem., 2011, vol. 76, p. 7377. doi 10.1021/jo201061f.
Suga, H., Adachi, Y., Fujimoto, K., Furihata, Y., Tsuchida, T., Kakehi, A., and Baba, T., J. Org. Chem., 2009, vol. 74, p. 1099. doi 10.1021/jo802392c
Suga, H., Funyu, A., and Kakehi, A., Org. Lett., 2007, vol. 9, p. 97. doi 10.1021/ol062675y
Suga, H., Nakajima, T., Itoh, K., and Kakehi, A., Org. Lett., 2005, vol. 7, p. 1431. doi 10.1021/ol050397h
Lu, Z., Wilsily, A., and Fu, G.C., J. Am. Chem. Soc., 2011, vol. 133, no. 22, p. 8154. doi 10.1021/ja203560q
Owston, N.A. and Fu, G.C., J. Am. Chem. Soc., 2010, vol. 132, p. no. 34, p. 11908. doi 10.1021/ja105924f
Saito, B. and Fu, G.C., J. Am. Chem. Soc., 2008, vol. 130, no. 21, p. 6694. doi 10.1021/ja8013677
Fossey, J.S., Matsubara, R., Vital, P., and Kobayashi, S., Org. Biomol. Chem., 2005, vol. 3, p. 2910. doi 10.1039/B505404D
Evans, D.A. and Seidel, D., J. Am. Chem. Soc., 2005, vol. 127, no. 28, p. 9958. doi 10.1021/ja052935r
Evans, D.A., Shizue, M., and Seidel, D., J. Am. Chem. Soc., 2007, vol. 129, no. 37, p. 11583. doi 10.1021/ja0735913
Reznikov, A.N. and Klimochkin, Y.N., Russ. J. Org. Chem., 2012, vol. 48, no. 12, p. 1526. doi 10.1134/S1070428012120056
Reznikov, A.N., Sybiryakova, A.E., Rybakov, V.B., and Klimochkin, Yu.N., Tetrahedron Asym., 2015, vol. 26, p. 1050. doi 10.1016/j.tetasy.2015.08.003
Reznikov, A.N., Sibiryakova, A.E., and Klimochkin, Yu.N., Russ. J. Gen. Chem., 2014, vol. 84, no. 11, p. 2280. doi 10.1134/S1070363214110437
Reznikov, A.N., Sibiryakova, A.E., and Klimochkin, Yu.N., Russ. J. Org. Chem., 2014, vol. 50, no. 11, p. 1695. doi 10.1134/S107042801411027X
Reznikov, A.N., Golovin, E.V., and Klimochkin, Yu.N., Russ. J. Org. Chem., 2013, vol. 49, no. 5, p. 663. doi 10.1134/S1070428013050047
Reznikov, A.N., Osyanin, V.A., and Klimochkin, Yu.N., Izv. Vuzov, Ser. Khim. Khin. Tekhnol., 2012, vol. 55, no. 12, p. 86.
Reznikov, A.N. and Klimochkin, Yu.N., RF Patent 2488576, 2013; Byull. Izobret., 2013, no. 21.
Reznikov, A.N., Sidnin, E.A., and Klimochkin, Yu.N., RF Patent 2529996, 2014; Byull. Izobret., 2014, no. 28.
Reznikov, A.N., Sidnin, E.A., and Klimochkin, Yu.N., RF Patent 2555370, 2015; Byull. Izobret., 2015, no. 19.
Reznikov, A.N., Sidnin, E.A., and Klimochkin, Yu.N., Russ. J. Org. Chem., 2013, vol. 49, no. 11, p. 1600. doi 10.1134/S1070428013110067
Sidnin, E.A., Reznikov, A.N., Shiryayev, V.A., and Klimochkin, Yu.N., Russ. J. Org. Chem., 2014, vol. 50, no. 11, p. 1579. doi 10.1134/S1070428014110074
Ihara, Y. and Tsuchiya, R., Bull. Chem. Soc. Japan, 1984, vol. 57, no. 10, p. 2829. doi 10.1246/bcsj.57.2829
Ihara, Y., Fukuda, Y., and Sone, K., Inorg. Chem., 1987, vol. 26, no. 22, p. 3745. doi 10.1021/ic00269a025
Yang, D., Li, D., Wang, L., Zhao, D., and Wang, R., J. Org. Chem., 2015, vol. 80, no. 9, p. 4336. doi 10.1021/acs.joc.5b00013
Meshkovaya, V.V., Yudashkin, A.V., Bushueva, P.Y., Eremeeva, N.B., and Klimochkin, Y.N., Tetrahedron, 2014, vol. 70, no. 19, p. 3211. doi 10.1016/j.tet.2014.02.092
Kusakov, M.M., Shimanko, N.A., and Shishkina, M.V., Ul’trafioletovye spektry pogloshheniya aromaticheskikh uglevodorodov (UV Absorption Spectra of Aromatic Hydrocarbons), Moscow: Akad. Nauk SSSR, 1963, p. 7.
Ihara, Y. and Toda, R., Thermochim. Acta, 1994, vol. 237, no. 1, p. 167. doi 10.1016/0040-6031(94)85196-4
Gonzalez, E., Rodrigue-Witchel, A., and Reber, C., Coord. Chem. Rev., 2007, vol. 251, p. 351. doi 10.1016/j.ccr.2006.08.011
Ihara, Y., Fukui, T., and Sone, K., Bull. Chem. Soc. Japan, 1986, vol. 59, no. 6, p. 1825. doi 10.1246/bcsj.59.1825
Cho, J., Lee, Y.-M., Kim, S.Y., and Nam, W., Polyhedron, 2010, vol. 29, p. 446. doi 10.1016/j.poly.2009.06.071
Moreno-Lara, B., Carabineiro, S.A., Krishnamoorthy, P., Rodriguez, A.M., Mano, J.F., Manzano, B.R., Jalon, F.A., and Gomes, P.T., J. Organomet. Chem., 2015, vols. 799–800, p. 90. doi 10.1016/j.jorganchem.2015.09.004
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © A.E. Sibiryakova, A.N. Reznikov, V.B. Rybakov, Yu.N. Klimochkin, 2016, published in Zhurnal Obshchei Khimii, 2016, Vol. 86, No. 11, pp. 1834–1840.
Rights and permissions
About this article
Cite this article
Sibiryakova, A.E., Reznikov, A.N., Rybakov, V.B. et al. Synthesis of Ni(II) complexes with chiral derivatives of cyclohexane-1,2-diamine, bycyclo[2.2.2]octane-2,3-diamine, and 1,2-diphenylethane-1,2-diamine. Russ J Gen Chem 86, 2477–2483 (2016). https://doi.org/10.1134/S107036321611013X
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S107036321611013X