Abstract
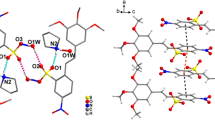
X-ray diffraction analysis established that 3-(4-N,N-dimethylaminophenyl)-2-nitro-1-phenylprop-2-en-1-one has E configuration both in crystal and in solution. The benzoyl group deviates from the styrene plane by 89.83°. The elongation of the C=C bond and shortening of its surrounding single bonds points to a high polarization of the molecule, implying a great contribution of the bipolar structure to the ground state.
Similar content being viewed by others
References
Karakhanov, R.A., Kelarev, V.I., and Polivin, Yu.N., Russ. Chem. Rev., 1993, vol. 62, no. 2, p. 169. doi 10.1070/RC1993v062n02ABEH000011
Giller, S.A., Zile, A.Ya., and Berklava, M. Ya., USSR Inventor’s Certificate 186635, 1966; Byull. Izobret., 1966, no. 19.
Berestovitskaya, V.M., Zobacheva, M.M., and Vasil’eva, O.S., Izv. Ross. Gos. Ped. Univ. im. A.I. Gertsena, 2002, no. 2, no. 4, p. 133.
Perekalin, V.V., Sopova, A.S, and Lipina, E.S., Nepredel’nye nitrosoedineniya (Unsaturated Nitro Compounds), Leningrad: Khimiya, 1982.
Perekalin, V.V., Lipina, E.S., Berestovitskaya, V.M., and Efremov, D.A., Nitroalkenes. Conjugated Nitrocompounds, Chichester: Wiley, 1994.
Ono, N., The Nitro Group in Organic Synthesis, New York: Wiley‒VCH, 2001. doi 10.1002/0471224480
Berestovitskaya, V.M., Baichurin, R.I., and Aboskalova, N.I., Sopryazhennye nitroeteny, geminal’no aktivirovannye slozhnoefirnoi, tsiano-i atsil’noi gruppami (Conjugated Nitroethenes Geminally Activated with Ester, Cyano, and Acyl Groups), St. Petersburg: Asterion, 2014.
Berestovitskaya, V.M., Baichurin, R.I., Aboskalova, N.I., Lysenko, K.A., Berkova, G.A., and Fel’gendler, A.V., Russ. J. Gen. Chem., 2009, vol. 79, no. 10, p. 2191. doi 10.1134/S1070363209100181
Paperno, T.Ya., Perekalin, V.V., and Sopova, A.S., J. Appl. Spectr., 1973, vol. 19, no. 4, p. 1299. doi 10.1007/BF00604072
Baichurin, R.I., Aboskalova, N.I., Trukhin, E.V., and Berestovitskaya, V.M., Russ. J. Gen. Chem., 2015, vol. 85, no. 8, p. 1845. doi 10.1134/S1070363215080101
Hamdellou, L., Hernandez, O., and Meinnel, J., Acta Crystallogr., Sect. C, 2006, vol. 62, no. 9, p. o557. doi 10.1107/S0108270106025819
Gupta, V.K. and Singh, R.A., RSC Adv., 2015, vol. 5, no. 48, p. 38591. doi 10.1039/C5RA04907E
Jasiński, R., Mirosław, B., Demchuk, O.M., Babyuk, D., and Łapczuk-Krygier, A., J. Mol. Struct., 2016, vol. 1108, p. 689. doi 10.1016/j.molstruc.2015.12.056
Chetkina, L.A., Popova, E.G., and Gol’der, G.A., J. Struct. Chem., 1974, vol. 15, no. 5, p. 768. doi 10.1007/BF00747284
Fonseca, I., Martinez-Carrera, S., Garcia-Blanco, S., Rodriguez, J.G., and Subirats, J.B., J. Cryst. Spectrosc. Res., 1988, vol. 18, no. 3, p. 265. doi 10.1007/BF01194317
Sonar, V.N., Parkin, S., and Crooks, P.A., Acta Crystallogr., Sect. C, 2005, vol. 61, no. 8, p. o527. doi 10.1107/S0108270105022298
Fel’gendler, A.V., Aboskalova, N.I., and Berestovitskaya, V.M., Russ. J. Gen. Chem., 2000, vol. 70, no. 7, p. 1087.
Bruker. APEX2 Software Suite for Crystallographic Programs, Madison, Wis.: Bruker AXS, 2009.
Bruker. Area Detector Control and Integration Software. Version 6.0. In: SMART and SAINT. Madison, Wis.: Bruker AXS, 2003.
Sheldrick, G.M., SADABS. Program for Absorption Correction, Göttingen, Germany: Univ. of Göttingen, 1997.
Sheldrick, G.M., Acta Crystallogr., Sect. A, 2008, vol. 64, no. 1, p. 112. doi 10.1107/S0108767307043930
Macrae, C.F., Edgington, P.R., McCabe, P., Pidcock, E., Shields, G.P., Taylor, R., Towler, M., and van de Streek, J., J. Appl. Crystallogr., 2006, vol. 39, no. 3, p. 453. doi 10.1107/S002188980600731X
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © V.M. Berestovitskaya, R.I. Baichurin, D.R. Islamov, O.N. Kataeva, N.I. Aboskalova, E.V. Trukhin, 2016, published in Zhurnal Obshchei Khimii, 2016, Vol. 86, No. 10, pp. 1664–1669.
Rights and permissions
About this article
Cite this article
Berestovitskaya, V.M., Baichurin, R.I., Islamov, D.R. et al. 3-(4-N,N-dimethylaminophenyl)-2-nitro-1-phenylprop-2-en-1-one by 13C NMR spectroscopy and X-ray diffraction analysis. Russ J Gen Chem 86, 2312–2317 (2016). https://doi.org/10.1134/S107036321610011X
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S107036321610011X