Abstract
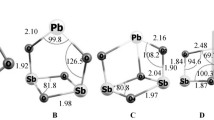
Reactions of gas-phase synthesis of lead phosphates and tellurates have been studied. Standard enthalpies of formation and atomization of gaseous PbPO2, PbPO3, PbP2O6, PbTeO3, and Pb2TeO4 salts have been determined.
Similar content being viewed by others
References
Lopatin, S.I., Shugurov, S.M., Panin, A.I., and Prikhod’ko, I.V., Russ. J. Gen. Chem., 2016, vol. 86, no. 4, p. 778. doi 10.1134/S1070363216040034
Kazenas, E.K. and Tsvetkov, Yu.V., Termodinamika ispareniya oksidov (Thermodynamics of Oxide Evaporation), Moscow: LKI, 2008.
Kazenas, E.K. and Tsvetkov, Yu.V., Isparenie oksidov (Oxide Evaporation), Moscow: Nauka, 1997.
Drowart, J., Colin, R., and Exteen, G., Trans. Faraday Soc., 1965, vol. 61, no. 511, p. 1376.
Chizhikov, D.M., Kazenas, E.K., and Tsvetkov, Yu.V., Izv. Akad. Nauk SSSR, Ser. Metally, 1969, no. 5, p. 57.
Kazenas, E.K. and Petrov, A.A., Metally, 1996, no. 4, p. 22.
Semenikhin, V.I., Rykov, A.N., and Sidorov, L.N., Russ. J. Phys. Chem., 1983, vol. 57, no. 7, p. 1008.
Popovic, A., Lesar, A., Gucek, M., and Bencze, L., Rapid Commun. Mass Spectrom., 1997, vol. 11, no. 5, p. 459. doi 10.1002/(SICI)10970231(199703)11:5<459::AID-RCM889>3.0.CO;2-G
Lopatin, S.I., Mittova, I.Ya., Gerasimov, F.S., Shugurov, S.M., Kostryukov, V.F., and Skorokhodova, S.M., Russ. J. Inorg. Chem., 2006, vol. 51, no. 10, p. 1646. doi 10.1134/S0036023606100214
Semenikhin, V.I., Sorokin, I.D., Yurkov, L.F., and Sidorov, L.N., Glass Phys. Chem., 1987, vol. 13, no. 4, p. 282.
Rat’kovskii, I.A., Kris’ko, L.Ya., Nalivaiko, A.G., and Shashkin, V.S., Izv Akad. Nauk BSSR, Ser. Khim., 1977, no. 6, p. 84.
Nalivaiko, A.G. and Ratkovskii, I.A., Inorg. Mater., 1981, vol. 17, no. 6, p. 840.
Nalivaiko, A.G., Smolyag, N.L.. and Ratkovskii, I.A., Khim. Khim. Tehnol., Minsk, 1981, no. 16, p. 46.
Nalivaiko, A.G., Cand. Sci. (Chem.) Dissertation, Minsk, 1983.
Nikolaev, E.N., Ovchinnikov, K.V., and Semenov, G.A., Russ. J. Gen. Chem., 1984, vol. 54, no. 5, p. 977.
Kunkel, K., Milke, E., and Binnewies, M., Int. J. Mass Spectr., 2014, vol. 374, p. 12. doi 10.1016/j.ijms.2014.09.019
Kunkel, K., Milke, E., and Binnewies, M., Dalton Trans., 2014, vol. 43, no. 14, p. 5401. doi 10.1039/c3dt53202j
Kunkel, K., Milke, E., and Binnewies, M., Eur. J. Inorg. Chem., 2015, vol. 2015, no. 1, p. 124. doi 10.1002/ejic.201402734
Energii razryva khimicheskikh svyazei. Potentsialy ionizatsii i srodstvo k elektronu (The Energies of Break Chemical Bonds. The Ionization Potentials and Electron Affinity), Kondrat’ev, V.N., Ed., Moscow: Nauka, 1974.
Semenov, G.A., Nikolaev, E.N., Kuligina, L.A., and Frantseva, K.E., Abstracts of Papers, Vsesoyuzn. konf. “Khimiya paroobraznykh neorganicheskikh soedinenii i protsessov paroobrazovaniya” (All-Union. Conf. “Chemical Vaporized Inorganic Compounds and Processes of Evaporation”), Minsk, 1976, p. 179.
Lopatin, S.I., Russ. J. Gen. Chem., 1997, vol. 67, no. 2, p. 193.
Lopatin, S.I. and Semenov G.A., Zh. Obshch. Khim., 1995, vol. 65, no. 7, p. 1060.
Lopatin, S.I. and Semenov, G.A., Russ. J. Gen. Chem., 1996, vol, no. 2, p. 170.
Termodinamicheskie svoistva individual’nykh veshchestv (Thermodynamic Properties of Individual Substances), Glushko, V.P., Ed., Moscow: Akad. Nauk SSSR, 1978–1984.
Muenow, D.W., Hastie, J.W., Hauge, R., Bautiste, R., and Margrave, J.L., Trans. Faraday Soc., 1969, vol. 65, p. 3210.
Lopatin, S.I., Semenov, G.A., and Shugurov, S.M., Russ. J. Gen. Chem., 2003, vol. 73, no. 2, p. 169. doi 10.1023/A:1024767332732
Zhao, Y. and Truhlar, D.G., Theor. Chem. Account., 2008, vol. 120, nos. 1–3, p. 215. doi 10.1007/s00214-007-0310-x
Moller, C. and Plesset, M.S., Phys. Rev., 1934, vol. 46, no. 7, p. 618. doi 10.1103/PhysRev.46.618
Bartlett, R.J. and Musial, M., Rev. Mod. Phys., 2007, vol. 79, no. 1, p. 291. doi 10.1103/RevModPhys.79.291
May, A.J. and Manby, F.R., J. Chem. Phys., 2004, vol. 121, no. 10, p. 4479. doi 10.1063/1.1780891
Werner, H.-J., Knowles, P.J., Knizia, G., Manby, F.R., and Schutz, M., MOLPRO, Version 2010.1, a Package of ab initio Programs. http://www.molpro.net
Werner, H.-J. and Manby, F.R., J. Chem. Phys., 2006, vol. 124, no. 5, p. 054114. doi 10.1063/1.2150817
Manby, F.R., Werner, H.-J., Adler, T.B., and May, A.J., J. Chem. Phys., 2006, vol. 124, no. 9, p. 094103. doi 10.1063/1.2173247
Werner, H.-J., J. Chem. Phys., 2008, vol. 129, no. 10, p. 101103. doi 10.1063/1.2982419
Werner, H.-J., Adler, T.B., and Manby, F.R., J. Chem. Phys., 2007, vol. 126, no. 16, p. 164102. doi 10.1063/1.2712434
Kniza, G., Adler, N.B., and Werner, H.-J., J. Chem. Phys., 2009, vol. 130, no. 5, p. 054104. doi 10.1063/1.3054300
Bross, D.H., Hill, J.G., Werner, H.-J., and Peterson, K.A., J. Chem. Phys., 2013, vol. 139, no. 9, p. 094302. doi 10.1063/1.4818725
Frisch, M.J., Trucks, G.W., Schlegel, H.B., Scuseria, G.E., Robb, M.A., Cheeseman, J.R., Scalmani, G., Barone, V., Mennucci, B., Petersson, G.A., Nakatsuji, H., Caricato, M., Li, X., Hratchian, H.P., Izmaylov, A.F., Bloino, J., Zheng, G., Sonnenberg, J.L., Hada, M., Ehara, M., Toyota, K., Fukuda, R., Hasegawa, J., Ishida, M., Nakajima, T., Honda, Y., Kitao, O., Nakai, H., Vreven, T., Montgomery, Jr., J.A., Peralta, J.E., Ogliaro, F., Bearpark, M., Heyd, J.J., Brothers, E., Kudin, K.N., Staroverov, V.N., Keith, T., Kobayashi, R., Normand, J., Raghavachari, K., Rendell, A., Burant, J.C., Iyengar, S.S., Tomasi, J., Cossi, M., Rega, N., Millam, J.M., Klene, M., Knox, J.E., Cross, J.B., Bakken, V., Adamo, C., Jaramillo, J., Gomperts, R., Stratmann, R.E., Yazyev, O., Austin, A.J., Cammi, R., Pomelli, C., Ochterski, J.W., Martin, R.L., Morokuma, K., Zakrzewski, V.G., Voth, G.A., Salvador, P., Dannenberg, J.J., Dapprich, S., Daniels, A.D., Farkas, O., Foresman, J.B., Ortiz, J.V., Cioslowski, J., and Fox, D.J. GAUSSIAN 09, Revision D.01. Gaussian, Inc., Wallingford CT., 2010.
Weigend, F. and Ahlrichs, R., Phys. Chem. Chem. Phys., 2005, vol. 7, no. 18, p. 3297. doi 10.1039/b508541a
NIST Chemistry Web Book, NIST Standard Reference Database Number 69. Gaithersburg: National Institute of Standards and Technology, 2014. http://webbook.nist.gov.
Chertihin, G.V. and Andrews, L., J. Chem. Phys., 1996, vol. 105, no. 7, p. 2561. doi 10.1063/1.472122
Lopatin, S.I., Shugurov, S.M., Panin, A.I., and Emelyanova, K.A., Russ. J. Gen. Chem., 2015, vol. 85, no. 6, p. 1351. doi 10.1134/S1070363215060018
Lopatin, S.I., Russ. J. Gen. Chem., 1999, vol. 69, no. 9, p. 1364.
Lopatin S.I., Russ. J. Gen. Chem., 2007, vol. 77, no. 11, p. 1823. doi 10.1134/S1070363207110011
Paule, R.C. and Mandel, J., Pure Appl. Chem., 1972, vol. 31, no. 3, p. 371.
Paule, R.C. and Mandel, J., Pure Appl. Chem., 1972, vol. 31, no. 3, p. 397.
Recent Development in Mass Spectrometry, Ogata, K. and Hayakawa, T., Eds., Baltimore: University Park Press, 1970, p. 814.
Guido, M. and Gigli, G., High Temp. Sci., 1975, vol. 7, no. 2, p. 122.
Sheer, M.D. and Fine, J., J. Chem. Phys., 1962, vol. 36, no. 6, p. 1647.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © K.A. Emelyanova, S.M. Shugurov, A.I. Panin, S.I. Lopatin, 2016, published in Zhurnal Obshchei Khimii, 2016, Vol. 86, No. 10, pp. 1591–1604.
Rights and permissions
About this article
Cite this article
Emelyanova, K.A., Shugurov, S.M., Panin, A.I. et al. Thermochemical study of gaseous salts of oxygen-containing acids: XXII.1 Lead salts. Russ J Gen Chem 86, 2243–2255 (2016). https://doi.org/10.1134/S1070363216100029
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070363216100029