Abstract
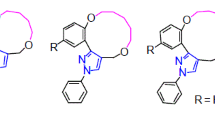
A series of new tricyclic macrocycles containing a chalcone moiety were synthesized from chalcones through alkylation using different dibromoalkanes. All the synthesized compounds were characterized by IR, 1H NMR, 13C NMR, and mass spectral data and evaluated for their in vitro antimicrobial activity.
Similar content being viewed by others
References
DeLorbe, J.E., Clements, J.H., Whiddon, B.B., and Martin, S. F., ACS Med. Chem. Lett., 2010, vol. 1, p. 448. DOI: 10.1021/ml100142y.
Madsen, C.M. and Clausen, M.H., Eur. J. Org. Chem., 2011, p. 3107. DOI: 10.1002/ejoc.201001715.
Halland, N., Blum, H., Buning C., Kohlmann, M., and Lindenschmidt, A., ACS Med. Chem. Lett., 2014, vol. 5, p. 193. DOI: 10.1021/ml4004556.
Jefferson, E.A., Arakawa, S., Blyn, L.B., Miyaji, A., Osgood, S.A., Ranken, R., Risen, L.M., and Swayze, E.E., J. Med. Chem., 2002, vol. 45, p. 3430.
Marsault, E. and Peterson, M.L., J. Med. Chem., 2011, vol. 54, p. 1961. DOI: 10.1021/jm1012374.
Obniska, J., Zeic, A., and Zagorska, A., Acta Pol. Pharm., 2002, vol. 59, no. 3, p. 209. ISSN 0001-6837.
Mukherjee, S., Kumar, V., Prasad, A.K., Raj, H.G., Bracke, M.E., Olsen, C.E., Jain, S.C., and Parmar, V.S., Bioorg. Med. Chem., 2001, vol. 9, p. 337. DOI: 10.1016/S0968-0896(00)00249-2.
Trivedi, J.C., Bariwal, J.B., Upadhyay, K.D., Naliapara, Y.T., Soshi, S.K., Pannecouque, C.C., De Clercq, E., and Shah, A.K., Tetrahedron Lett., 2007, vol. 48, p. 8472. DOI: 10.1016/j.tetlet.2007.09.175.
Satyanarayana, M., Tiwari, P., Tripathi, B. K., Srivastava, A.K., and Pratap. R., Bioorg. Med. Chem., 2004, vol. 12, p. 883. DOI: 10.1016/j.bmc.2003.12.026.
Go, M.L., Wu, X., and Liu, X.L., Curr. Med. Chem., 2005, vol. 12, p. 483.
Hsieh, H.K., Tsao, L.T., Wang, J.P., and Lin, C.N., J. Pharm. Pharmacol., 2000, vol. 52, no. 2, p. 163. DOI: 10.1211/0022357001773814.
Dongamanti, A., Aamate, V.K., Devulapally, M.G., Gundu, S., Kotni, M.K., Manga, V., Sridhar, B., and Prasad, E., Bioorg. Med. Chem.Lett., 2015, vol. 25, p. 898. DOI: 10.1016/j.bmcl.2014.12.066.
Raval, A.A. and Shah, N.M., J. Org. Chem., 1956, vol. 21, p. 1408. DOI: 10.1021/jo01118a021.
Rao, Y.K., Fang, S.H., and Tzeng, Y.M., Bioorg. Med. Chem., 2004, vol. 12, p. 2679. DOI: 10.1016/j.bmc.2004.03.014.
Radha, K., Pritam, T., Han, Y.Y., Tara, M.K., Park, P.H., Youngwha, N., Eunyoung, L., Jeon, K.H., Cho, W.J., Heesung, C., Youngjoo, K., and Lee, E.S., Eur. J. Med. Chem., 2012, vol. 49, p. 219. DOI: 10.1016/j.ejmech.2012.01.015.
Rina, M., Tapas, K.M., and Ashok K.M., Arkivoc, 2012, vol. 9, p. 95. DOI: 12-7621BP.
Author information
Authors and Affiliations
Corresponding author
Additional information
The text was submitted by the authors in English.
Rights and permissions
About this article
Cite this article
Dongamanti, A., Aamate, V.K., Gundu, S. et al. Synthesis and antimicrobial evaluation of tricyclic macrocycles containing a chalcone moiety. Russ J Gen Chem 86, 1705–1710 (2016). https://doi.org/10.1134/S1070363216070288
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070363216070288