Abstract
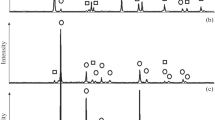
Precipitation of titanium dioxide layers from the gas phase in the reaction system containing titanium tetraisopropylate and oxygen at the total pressure 1 kPa is studied. It is shown that in the range of 300–500°C the precipitation proceeds in the kinetic regime and is accompanied by the formation of layers of monotonous thickness containing nanocrystalline phases of anatase and rutile. In the temperature range 300–350°C the activation energy value was 92.7 kJ mol−1, and at higher temperatures (up to 500°C) it decreased to 17.5 kJ mol−1. The increase in the precipitation temperature caused the increase in relative amount of rutile in the precipitated layers.
Similar content being viewed by others
References
Depero, L.E., Ferroni, M., Giudi, V., Marca, G., Martinelli, G., Nelli, P., Sangaletti, L., and Sberveglieri, G., Sensors and Actuatirs, B., 1996, nos. 35–36, p. 381.
Pore, V., Rahtu, A., Leskela, M., Ritala, M., Sajavaara, T., and Keinonen, J., Chem. Vap. Deposition, 2004, vol. 10, no. 3, p. 143.
Xie, Q., Deduytsche, D., Schaekers, M., Caymax, M., Delabie, A., Qu, X.-P., and Detavernier, C., Appl. Polym. Lett., 2010, no. 97, p. 112905.
Gratzel, M., J. So—Gel Sci. Technol., 2001, no. 22, p. 7.
Taylor, C.J., Gilmer, D.C., Colombo, D.G., Wilk, G.D., Campbell, S.A., Roberts, J., and Gladfelter, W.L., J. Am. Chem. Soc., 1999, vol. 121, p. 5220.
Aleksandrov, S.E., Filatov, L.A., Baryshnikova, M.V., and Andreeva, V.D., Zh. Obshch. Khim., 2010, vol. 80, no. 6, p. 1015.
Jogi, I., Pars, M., Aarik, J., Aidla, A., Laan, M., Sundqvist, J., Oberbeek, L., Heitmann, J. and Kukli, K., Thin Solid Films, 2008, vol. 516, p. 4855.
Busani, T. and Devine, R.A.B., Semicond. Sci. Technol., 2005, vol. 20. p. 870.
Stranski, I., Krastanow Von, L., Akad. Wiss. Lit. Mainz Math.-Natur. Kl. IIb, 1939, vol. 146, p. 797.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © M.V. Baryshnikova, S.E. Aleksandrov, L.A. Filatov, A.B. Berberov, 2013, published in Zhurnal Obshchei Khimii, 2013, Vol. 83, No. 8, pp. 1367–1371.
Rights and permissions
About this article
Cite this article
Baryshnikova, M.V., Aleksandrov, S.E., Filatov, L.A. et al. Kinetic rules of precipitation of thin films of titanium dioxide from the gas phase containing titanium tetraisopropylate. Russ J Gen Chem 83, 1596–1600 (2013). https://doi.org/10.1134/S1070363213080215
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070363213080215