Abstract
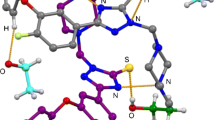
The formation by 1H-1,2,4-triazoline-3-thiones in dilute chloroform solution of n-σ*-complex with molecular iodine of the composition C2H2N3S·I2 was studied by electronic spectroscopy in the UV and visible regions (log β = 2.14±0.05). By the XRD method the crystal and molecular structure of the product of chemical interaction of the thione with molecular iodine in ethanol, 1,2-di(1H-1,2,4-triazol-3-yl) disulfide, was determined.
Similar content being viewed by others
References
Mekuskiene, G., Gaidelis, P., and Vainilavicius, P., Pharmazie, 1998, vol. 53, no. 2, p. 94.
Sahin, G., Palaska, E., Kelicen, P., Demirdamar, R., and Altmok, G., Arzneim. Forsch., 2001, vol. 51, p. 478.
Eweiss, N.F., Bahajaj, A.A., and Elsherbini, E.A., J. Heterocycl. Chem., 1986, vol. 23, p. 1451.
Mazzone, G., Bonina, F., Arrigo Reina, R., and Blandino, G., Farmaco, 1981, vol. 36, p. 181.
Knight, P.D., Demauriac, R.A., and Graham, P.A., Germany Patent 2.811.025, 1978; C. A., 1979, vol. 90, p. 79146s.
Dobosz, M., Acta Polon. Pharm., 1984, vol. 41, p. 43.
Dwyer, F.P. and Mellor, D.P., Chelating Agents and Metal Chelates, New York: Academic Press, 1964.
Tolpygin, I.E., Shepelenko, E.N., Borodkin, G.S., Dubonosov, A.D., Bren’, V.A., and Minkin, V.I., Chem. Heterocycl. Compd., 2010, vol. 46, no. 5, p. 542.
Comprehensive Heterocyclic Chemistry I, Katritzky, A.R. and Rees, Ch.W., Eds., 1984, vol. 5, pt. 4A, p. 733.
Comprehensive Heterocyclic Chemistry II, Katritzky, A.R., Rees, Ch.W., and Scriven, E.F.V., Eds., 1996, vol. 4, p. 127.
Prauda, I. and Reiter, J., J. Heterocycl. Chem., 2003, vol. 40, no. 5, p. 821.
Daga, V., Hadajikakou, S.K., Hadjiliadis, N., Kubicki, M., dos Santos, J.H.Z., and Butler, I.S., Eur. J. Inorg. Chem., 2002, p. 1718.
Franklyn, J., Clinical Medicine, 2003, vol. 3, no. 1, p. 11.
Dongsheng, Liu, Yaping, Xu, Xinfa, Li, Shaoming, Ying, and Wentong, Chen, Acta Cryst. (E), 2008, vol. 64, p. o247.
Gordon, A.J. and Ford, R.A., The Chemist’s Companion. A Handbook of Practical Data, Techniques and References, New York: Wiley, 1972.
Sheldrick, G.M., SADABS v.2.01, Bruker/Siemens Area Detector Absorption Correction Program, Bruker AXS, Madison, Wisconsin, USA, 1998a.
Sheldrick, G.M., Programs SHELXS97 (Crystal Structure Solution) and SHELXL97 (Crystal Structure Refinement), Germany, University of Göttingen, 1997.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © M.S. Chernovyants, N.V. Aleshina, I.N. Shcherbakov, Z.A. Starikova, 2013, published in Zhurnal Obshchei Khimii, 2013, Vol. 83, No. 5, pp. 852–854.
Rights and permissions
About this article
Cite this article
Chernovyants, M.S., Aleshina, N.V., Shcherbakov, I.N. et al. Spectroscopic study of interaction of 1H-1,2,4-triazoline-3-thione with molecular iodine. Russ J Gen Chem 83, 986–988 (2013). https://doi.org/10.1134/S1070363213050186
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070363213050186