Abstract
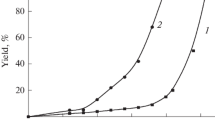
The cycloaddition reaction between 2,3-dimethylbuta-1,3-diene and allyl methacrylate proceeds by the second order kinetics. The rate constants increase with the increase in the excess of one of the reactants. The change in the effective rate constants is described by the Michaelis-Menten equation indicating that the reaction proceeds through the initial equilibrium stage of formation of an intermediate complex which then transforms into the product. The effective rate constants, the equilibrium constants of formation of the intermediate complex, and the rate constant of its transformation into the reaction product were determined, as well as the thermodynamic parameters of the formation of the intermediate complex and the activation parameters of the transformation of the intermediate complex into the product. The limiting stage of the reaction is established and its mechanism is suggested.
Similar content being viewed by others
References
Velazquez, J.M., Hertenstein, S.R., Clare, J.R., and Green, M.N., US Patent 2010/0152083, 2010.
Smets, J., Newman, M., Schenk, H., and Haspeter, P., US Patent 2008/0200359, 2008.
Pol’ova, I.S., Polyuzhin, I.P., Marshalok, G.O., and Gladii, A.I., Abstract of Papers, III Ukrainian Sci. Conf. of Students and Aspirants “Khimichny Karazinski Chitaniya 2011,” Kharkov, 2011, p. 41.
Shmid, R. and Sapunov, V.N., Neformal’naya kinetika (Unformal Cinetics), Moscow: Mir, 1985.
Jasinski, R., Kwiatnowska, M., and Baranski, A., Wiadom. Chemiczne, 2007, vol. 61, nos. 7–8, p. 485.
Woodward, R.B. and Katz, T.J., Tetrahedron, 1959, vol. 5, no. 1, p. 70.
Ross, S.D. and Kuntz, J., J. Am. Chem. Soc., 1954, vol. 76, no. 12, p. 3000.
Hammett, L., Physical Organic Chemistry, New York: McGraw-Hill, 1970.
Marshalok, G.A., Oglashennyi, Yu.I., Makitra, R.G., and Yatchishin, I.I., Zh. Org. Khim., 2003, vol. 39, no. 9, p. 1298.
Makitra, R.G., Tsikanchuk, Ya.M., and Turkevich, O.E., Dokl. Akad. Nauk SSSR, 1976, no. 5, p. 435.
Corsico Coda, A., Desimoni, G., Ferrari, E., Righetti, P., and Tacconi, G., Tetrahedron, 1984, vol. 40, no. 9, p. 1611.
Desimoni, G., Faita, G., Righetti, P., and Tacconi, G., J. Chem. Soc., Perkin Trans. 2, 1989, no. 3, p. 437.
Blankenburg, B., Fiedler, H., Hampelm, M., Hauthal, H., Just, G., and Kahlert, K., J. Prakt. Chem., 1974, vol. 316, no. 5, p. 804.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © I.S. Polevaya, G.G. Makitra, G.A. Marshalok, Ya.P. Kovalskyi, 2012, published in Zhurnal Obshchei Khimii, 2012, Vol. 82, No. 12, pp. 2016–2020.
Rights and permissions
About this article
Cite this article
Polevaya, I.S., Makitra, G.G., Marshalok, G.A. et al. Effect of the reactants molar ratio on the kinetics of cycloaddition of 2,3-dimethylbuta-1,3-diene to allyl methacrylate. Russ J Gen Chem 82, 1970–1974 (2012). https://doi.org/10.1134/S1070363212120109
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070363212120109