Abstract
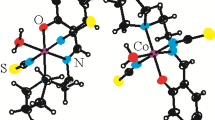
Salicylic, 5-chloro-5-bromo-5-nitro-, 3-methoxysalicylic, 2-hydroxy-1-naphthoic aldehydes and 2,3-, 2,4-, and 2,5-dihydroxybenzaldehyde were shown to react in ethanol with 2-(2-aminoethylamino)ethanol in the presence of copper acetate hydrate forming coordination compounds Cu(L1-9)CH3COO {HL1-8, 2-([2-(2-hydroxyethylamino)ethylimino]methyl}phenol (HL1) and respective chloro(HL2), bromo- (HL3), nitro- (HL4), methoxy- (HL8), or hydroxy-substituted (HL5-7); HL9, 2-{[2-(2-hydroxyethylamine)ethylimino] methylnaphthol)}. Structure of the complex Cu(L3)CH3COO was determined by X-ray diffraction analysis. Coordination polyhedron of its central atom is a distorted tetragonal bipyramid with (4+1+1) modes of coordinating the copper atom. The bipyramid base is formed by the atoms of phenol oxygen and of azomethine and imine nitrogen atoms of the ligand (HL3) and the oxygen atom of the acetate ion. Axial apices of the bipyramid are occupied by the alcohol oxygen atoms of the azomethine (HL3) and the second oxygen atom of the acetate ion. Other complexes are also of monomer structure. Azomethines (HL1-9) behave as monodeprotonated tetradentate O,N,N,O ligands. The thermolysis of substances includes a stage of complete thermal decomposition (360–530°C). Synthesized complexes show selective antimicrobial activity against a series of standard strains of Staphylococcus aureus and E. coli at the concentration in the range of 75–300 μg ml−1.
Similar content being viewed by others
References
Mashkovskii, M.D., Lekarstvennye sredstva (Medicines), Mosow: Novaya Volna, 2008.
Zhungietu, G.I. and Granik, V.G., Osnovnye printsipy konstruirovaniya lekarstv (Basic Principles of Constructing Drugs), Kishinev: IPK Mold. Gos. Univ., 2000.
Chumakov, Yu.M., Tsapkov, V.I., Petrenko, P.A., Popovski, L.G., Simonov, Yu.A., Bochelli, G., and Gulya, A.P., Kristallografiya, 2009, vol. 54, no. 2, p. 276.
Chumakov, Yu.M., Tsapkov, V.I., Filippova, I.G., Bochelli, G., and Gulya, A.P., Kristallografiya, 2008, vol. 53, no. 4, p. 669.
Chumakov, Yu.M., Tsapkov, V.I., Bochelli, G., Antosyak B. Ya., and Gulya, A.P., Zh. Strukt. Khim., 2006, vol. 47, no. 2, p. 352.
Chumakov, Yu.M., Tsapkov, V.I., Bochelli, G., Neiburger, M., and Gulya, A.P., Koord. Khim., 2006, vol. 32, no. 10. s. 775.
Samus’, N.M., Shlyakhov, E.N., Siba Kulemu, Burdenko, T.A., Chaika, T.S., Tsapkov, V.I., and Popov, M.S., Khim.-Farm. Zh., 1987, vol. 21, no. 4, p. 446.
Zelenin, K.N., Kuznetsova, O.B., Saminskaya, A.G., Alekseeva, V.V., Karimov, Z.T., Sivolodskii, E.P., Sofronov, G.A., Novikov, N.I., and Preobrazhenskaya, T.N., Khim.-Farm. Zh., 1994, vol. 31, no. 2, p. 34.
Gerbeleu, N.V., Arion, V.B., and Burges, J., Template Synthesis of Macrocyclic Compounds, Weinheim: Wiley-VCH, 1999.
Samus’, N.M., Tsapkov, V.I., Voitsekhovskaya, O.M., Burdenko, T.A., and Buracheva, S.A., Khim.-Farm. Zh., 1993, vol. 27, no. 12, p. 31.
Tsapkov, V.I., Popov, M.S., Fam Ngok Fung, and Samus’, N.M., Zh. Obshch. Khim., 1994, vol. 64, no. 4, p. 634.
Mikuriya, M. and Matsunami, K., Materials Science-Poland, 2005, vol. 23, no. 3, p. 773.
Nakamoto, K., Infrared and Raman Spectra of Inorganic and Coordination Compounds, Moscow: Mir, 1991, p. 257.
CrysAlisPro, Oxford Diffraction Ltd., Version 1.171.33.52 (release 06-11-2009 CrysAlis171.NET).
Sheldrick, G.M., SHELX-97. Program for the Refinement of Crystal Structure, University of Göttingen, Germany, 1997.
Clark, R.C. and Reid, J.S., Acta Cryst. A, 1995, vol. 51, p. 887.
Spek, A.L., J. Appl. Cryst., 2003, vol. 36, p. 7.
Pershin, G.N., Metody eksperimental’noi khimioterapii (Methods of Experimental Chemotherapy), Moscow: Meditsina, 1971, p. 357.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © A.P. Gulya, Yu.M. Chumakov, V.I. Tsapkov, V.O. Graur, K.S. Lozan-Tyrshu, E. Janno, B.Ya. Antosyak, V.F. Rudik, 2011, published in Zhurnal Obshchei Khimii, 2011, Vol. 81, No. 9, pp. 1521–1528.
Rights and permissions
About this article
Cite this article
Gulya, A.P., Chumakov, Y.M., Tsapko, V.I. et al. Synthesis, structure, and properties of coordination compounds of copper(II) acetate with substituted 2-{[2-(2-hydroxyethylamino)ethylimino]methyl}phenol. Russ J Gen Chem 81, 1859–1866 (2011). https://doi.org/10.1134/S1070363211090209
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070363211090209