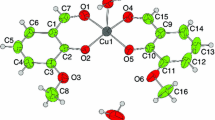
Abstract
The processes and products of the reaction of copper(II) sulfide and oxide with ammonium chloride were studied. The process of the copper(II) compounds hydrochlorination was investigated with thermogravimetry and kinetic studies. Reaction products were studied by infrared spectroscopy and X-ray phase analysis.
Similar content being viewed by others
References
Karapet’yants, M.Kh. and Drakin, S.I., Obshchaya i neorganicheskaya khimiya (General and Inorganic Chemistry), Moscow: Khimiya, 1992.
Kraidenko, R.I., Khim. Prom. Segodnya, 2008, no. 11, p. 13.
D’yachenko, A.N., Tsvetnye Metally, 2005, nos. 5–6, p. 71.
Wendlandt, W.W., Thermal Methods of Analysis, Moscow: Mir, 1978.
Delmont, B., Introduction a la cinetique Heterogene, Moscow: Mir, 1972.
Nakamoto, K., Infrared and Raman Spectra of Inorganic and Coordination Compounds: Applications in Coordination, Organometallic, and Bioinorganic Chemistry, Moscow: Mir, 1991.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © V.A. Borisov, A.N. D’yachenko, R.I. Kraidenko, 2011, published in Zhurnal Obshchei Khimii, 2011, Vol. 81, No. 7, pp. 1080–1082.
Rights and permissions
About this article
Cite this article
Borisov, V.A., D’yachenko, A.N. & Kraidenko, R.I. Reaction of ammonium chloride with the copper(II) sulfide and oxide, and identification of the reaction products. Russ J Gen Chem 81, 1430–1433 (2011). https://doi.org/10.1134/S107036321107005X
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S107036321107005X