Abstract
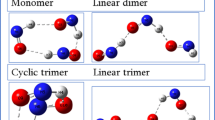
The structure and stability of heteromolecular van der Waals clusters (N2) n CO m ( n = 1–7; m = 1–3) was studied using ab initio MP2(full)/6-311+G* and CCSD(full)/6-311+G* methods. For clusters with (n + m) > 3 the polyhedron structures are the most preferable, whose stability increases with the number of interacting molecules. Incorporation of CO molecules results in weakening of binding in the cluster and lowering the stereochemical rigidity relative to homomolecular systems. Increase of percentage of CO is followed by a decrease of stability of the clusters.
Similar content being viewed by others
References
Hobza, P. and Zahradník, R., Intermolecular Complexes. The Role of van der Waals Systems in Physical Chemistry and in the Biodisciplines, Prague: Academia, 1988.
Nemukhin, A.V., Ross. Khim. Zh. (Zh. Ross. Khim. Obva im. D.I. Mendeleeva)., 1996, vol. 40, no. 3, p. 48.
Tang, J. and McKellar, A.R.W., J. Chem. Phys., 2003, vol. 119, no. 2, p. 754.
Long, C.A., Henderson, G., and Ewing, G.E., Chem. Phys., 1973, vol. 2, no. 4, p. 485.
Thakkar, A.J. and Smith, V.H.Jr., Mol. Phys., 1975, vol. 29, no. 3, p. 731.
Carnovale, F., Peel, J.B., and Rothwell, R.G., J. Chem. Phys., 1988, vol. 88, no. 2, p. 642.
Vanden Bout, P.A., Steed, J.M., Bernstein, L.S., and Klemperer, W., Astrophys. J., 1979, vol. 234, no. 2, p. 503.
Xu, Y. and Jäger, W., J. Chem. Phys., 1999, vol. 111, no. 13, p. 5754.
Kawashima, Y. and Nishiza, K., Chem. Phys. Lett., 1996, vol. 249, nos. 1–2, p. 87.
Xu, Y. and McKellar, A.R.W., J. Chem. Phys., 1996, vol. 104, no. 7, p. 2488.
Colbourn, E.A. and Douglas, A.E., J. Chem. Phys., 1976, vol. 65, no. 5, p. 1741.
Jäger, W. and Gerry, M.C.L., Chem. Phys. Lett., 1992, vol. 196, nos. 3–4, p. 274.
Böhm, H. and Ahlrichs, R., Mol. Phys., 1985, vol. 55, no. 5, p. 1159.
Avoird, A., Wormer, P.E.S., and Jansen, A.P.J., J. Chem. Phys., 1986, vol. 84, no. 3, p. 1629.
LeSar, R. and Shaw, M.S., J. Chem. Phys., 1986, vol. 84, no. 10, p. 5479.
Bohr, J.E. and Hunt, K.L.C., J. Chem. Phys., 1987, vol. 87, no. 7, p. 3821.
Mack, H. and Oberhammer, H., J. Chem. Phys., 1987, vol. 87, no. 4, p. 2158.
Pol, A., Avoird, A., and Wormer, P.E.S., J. Chem. Phys., 1990, vol. 92, no. 12, p. 7498.
Brookes, M.D. and McKellar, A.R.W., J. Chem. Phys., 1999, vol. 111, no. 6, p. 7321.
Roth, D.A., Surin, L.A., Dumesh, B.S., Winnewisser, G., and Pak, I., J. Chem. Phys., 2000, vol. 113, no. 8, p. 3034.
Xu, Y., Jäger, W., Surin, L.A., Pak, I., Panfilov, L.A., and Winnewisser, G., J. Chem. Phys., 1999, vol. 111, no. 23, p. 10476.
Fernández, B., Koch, H., and Makarewicz, J., J. Chem. Phys., 1999, vol. 110, no. 7, p. 8525.
Plönjes, E., Palm, P., Lee, W., Chidley, M.D., Adamovich, I.V., Lempert, W.R., and Rich, J.W., Chem. Phys., 2000, vol. 260, no. 3, p. 353.
Plönjes, E., Palm, P., Lee, W., Lempert, W.R., and Adamovich, I.V., J. Appl. Phys., 2001, vol. 89, no. 11, p. 5911.
Lee, W., Adamovich, I.V., and Lempert, W.R., J. Chem. Phys. 2001, vol. 114, no. 3, p. 1178.
Gribanova, T.N., Milov A.A., Starikov A.G., Gapurenko O.A., Gurashvili, V.A., Minyaev, R.M., and Minkin, V.I., Russ. Chem. Bull. Engl. Transl., 2008, vol. 57, no. 10, p. 2037.
Foresman, J.B. and Frisch, E., Exploring Chemistry with Electronic Structure Methods, Pittsburg: Gaussian Inc., 1996, 2nd ed.
Frisch, M.J., Trucks, G.W., Schlegel, H.B., Scuseria, G.E., Robb, M.A., Cheeseman, J.R., Montgomery, J.A., Jr., Vreven, T., Kudin, K.N., Burant, J.C., Millam, J.M., Iyengar, S.S., Tomasi, J., Barone, V., Mennucci, B., Cossi, M., Scalmani, G., Rega, N., Petersson, G.A., Nakatsuji, H., Hada, M., Ehara, M., Toyota, K., Fukuda, R., Hasegawa, J., Ishida, M., Nakajima, T., Honda, Y., Kitao, O., Nakai, H., Klene, M., Li, X., Knox, J.E., Hratchian, H.P., Cross, J.B., Adamo, C., Jaramillo, J., Gomperts, R., Stratmann, R.E., Yazyev, O., Austin, A.J., Cammi, R., Pomelli, C., Ochterski, J.W., Ayala, P.Y., Morokuma, K., Voth, G.A., Salvador, P., Dannenberg, J.J., Zakrzewski, V.G., Dapprich, S., Daniels, A.D., Strain, M.C., Farkas, O., Malick, D.K., Rabuck, A.D., Raghavachari, K., Foresman, J.B., Ortiz, J.V., Cui, Q., Baboul, A.G., Clifford, S., Cioslowski, J., Stefanov, B.B., Liu, G., Liashenko, A., Piskorz, P., Komaromi, I., Martin, R.L., Fox, D.J., Keith, T., Al-Laham, M.A., Peng, C.Y., Nanayakkara, A., Challacombe, M., Gill, P.M.W., Johnson, B., Chen, W., Wong, M.W., Gonzalez, C., and Pople, J.A., Gaussian-03. Rev., B.03, Pittsburgh (Pa): Gaussian Inc., 2003.
Hehre, W.J., Radom, L., Schleyer, P.v.R., and Pople, J.A., Ab Initio Molecular Orbital Theory, New York: Wiley, 1986.
Schaftenaar, G., Molden 3.4. CAOS/CAMM Center, The Netherlands, 1998.
Franken, K.A. and Dykstra, C.E., J. Phys. Chem., 1993, vol. 97, no. 44, p. 11408.
Xia, C., McKellar, A.R.W., and Xu, Y., J. Chem. Phys. 2000, vol. 113, no. 2, p. 525.
Xu, Y. and Jäger, W., J. Chem. Phys. 2000, vol. 113, no. 2, p. 514.
Surin, L.A., Potapov, A.V., Műller, H.S.P., Panfilov, V.A., Dumesh, B.S., Giesen, T.F., and Schlemmer, S., J. Mol. Struct., 2006, vol. 795, nos. 1–3, p. 198.
Fiser, J. and Polak, R., Chem. Phys. Lett., 2002, vol. 360, nos. 5–6, p. 565.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © T.N. Gribanova, A.A. Milov, O.A. Gapurenko, A.G. Starikov, V.A. Gurashvili, R.M. Minyaev, V.I. Minkin, 2011, published in Zhurnal Obshchei Khimii, 2011, Vol. 81, No. 5, pp. 719–731.
Rights and permissions
About this article
Cite this article
Gribanova, T.N., Milov, A.A., Gapurenko, O.A. et al. Structure and stability of the mixed polymolecular complexes of nitrogen and carbon nonooxide: A quantum chemical study. Russ J Gen Chem 81, 807–818 (2011). https://doi.org/10.1134/S1070363211050033
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070363211050033