Abstract
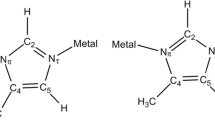
The equilibrium geometry and energy parameters of the complexes of Ca2+ and Mg2+ with 5-methyl-2-thioxotiazolidin-4-one (methylidene rhodanine) and its anion in a 1:1 ratio in different conformations were calculated by the quantum-chemical method with the density functional theory on the level of hybrid functional B3LYP in the basis of atomic orbitals 6–31+G(d). The influence of metal ion size on the number of possible isomeric coordinations was indicated. The principles of stabilization and destabilization of the structures depending on their conformations al structure were described. Based on the calculated equilibrium geometry parameters of the complexes conformations the effect of complexation on the structure of rhodanine ligand was elucidated. In the framework of a polarizable continuum the relative stability of the possible tautomeric forms of methylidene rhodanine in water was investigated. A new structure of the methylidene rhodanine anion distinquished by a specific distribution of negative charge is suggested.
Similar content being viewed by others
References
Bryk, R., Gold, B., Venugopal, A., Singh, J., Sam, y R., Pupek, K., Cao, H., Popescu, C., Gurney, M., Hotha, S., Cherian, J., Rhee, K., Ly, L., Converse, P.J., Ehrt, S., Vandal, O., Jiang, X., Schneider, J., Lin, G., and Nathan, C., Cell Host & Microbe, 2008, vol. 3, no. 3, p. 137.
Sortino, M., Delgado, P., Juárez, S., Quiroga, J., Abonía, R., Insuasty, B., Nogueras, M., Rodero, L., Garibotto, F.M., Enrize, R.D., and Zacchinoa, S.A., Bioorg. & Med. Chem., 2007, vol. 15, no. 1, pp. 484–494.
Orchard, M.G., Neuss, J.C., Galley, C.M.S., Carr, A., Porter, D.W., Smith, P., Scopes, D.I.C., Haydon, D., Vousden, K., Stubberfield, C.R., Young, K., and Page, M., Bioorg. & Med. Chem. Lett., 2004, vol. 14, no. 15, pp. 3975–3978.
Dolezel, J., Hirsova, P., Opletalova, V., Dohnal, J., Marcela, V., Kunes, J., and Jampilek, J., Molecules, 2009, vol. 14, no. 10, pp. 4197–4212.
Lesyk, R.B., Zimenkovsky, B.S., and Troc’ko, N.Y., Ukrainica Bioorganica Acta, 2004, vol. 1, pp. 29–38.
Russell, A.J., Westwood, I.M., Crawford, M.H.J., Robinson, J., Kawamura, A., Redfield, C., Laurieri, N., Lowe, E.D., Davies, S.G., and Sim, E., Bioorg. and Med. Chem., 2009, vol. 17, no. 2, pp. 905–918.
Verma, A. and Saraf, S.K., Europp. J. Med. Chem., 2008, vol. 43, pp. 897–905.
Irvine, M.W., Patrick, G.L., Kewney, J., Hastings, S.F., and MacKenzie, S.J., Bioorg. & Med. Chem. Lett., 2008, vol. 18, no. 6, pp. 2032–2037.
Loncharich, R.J., Nissen, J.S., and Boyd, D.B., Struct. Chem., 1996, vol. 7, no. 1, pp. 37–49.
Fabretti, A.C., Franchini, G., Peyronel, G., and Ferrari, M., Polyhedron, 1982, vol. 1, nos. 7–8, pp. 633–635.
Zaki, M.T.M., Abdel-Rahman, R.M., and El-Sayed, A.Y., Anal. Chim. Acta, 1995, vol. 307, pp. 127–138.
Tang, E., Yang, G., and Yin, J., Spectrochimica Acta, Part A, 2003, vol. 59, no. 3, pp. 651–656.
Boyd, D.B., J. Mol. Struct.: Theochem, 1997, vol. 401, no. 3, pp. 227–234.
Dewar, M.J.S. and Theil, W., J. Am. Chem. Soc., 1977, vol. 99, no. 15, pp. 4899–4907.
Stewart, J.J.P., J. Comp. Chem., 1989, vol. 10, no. 2, pp. 209–220.
Becke, A.D., J. Chem. Phys., 1993, vol. 98, no. 7, pp. 5648–5652.
Lee, C., Yang, W., and Parr, R.G., Phys. Rev., B, 1988, vol. 37, no. 2, pp. 785–789.
Francl, M.M., Petro, W.J., Hehre, W.J., Binkley, J.S., Gordon, M.S., DeFrees, D.J., and Pople, J.A., J. Chem. Phys., 1982, vol. 77, no. 7, pp. 3654–3665.
Krishnan, R., Binkley, J.S., Seeger, R., and Pople, J.A., J. Chem. Phys., 1980, vol. 72, no. 1, pp. 650–654.
Frisch, M.J., Trucks, G.W., Schlegel, H.B., Scuseria, G.E., Robb, M.A., Cheeseman, J.R., Montgomeri, J.A Jr., Vreven, T., Kudin, K.N., Burant, J.C., Millam, J.M., Iyengar, S.S., Tomasi, J., Barone, V., Mennucci, B., Cossi, M., Scalmani, G, Rega, N., Petersson, G.A., Nakatsuji, H., Hada, M., Ehara, M., Toyota, K., Fukuda, R., Hasegawa, J., Ishida, M., Nakajima, T., Honda, Y., Kitao, O., Nakai, H., Klene, M., Li, X., Knox, J.E., Hratchian, H.P., Cross, J.B., Adamo, C., Jaramillo, J., Gomperts, R., Stratmann, R.E., Yazyev, O., Austin, A.J., Cammi, R., Pomelli, C., Ochterski, J.W., Ayala, P.Y., Morokuma, K., Voth, G.A., Salvador, P., Dannenberg, J.J., Zakrzewski, V.G., Dapprich, S., Daniels, A.D., Strain, M.C., Farkas, O., Malick, D.K., Rabuck, A.D., Raghavachari, K., Foresman, J.B., Ortiz, J.V., Cui, Q., Baboul, A.G., Clifford, S., Cioslowski, J., Stefanov, B.B., Liu, G., Liashenko, A., Piskorz, P., Komaromi, I., Martin, R.L., Fox, D.J., Keith, T., Al-Laham, M.A., Peng, C.Y., Nanayakkara, A., Challacombe, M., Gill, P.M.W., Johnson, B., Chen, W., Wong, M.W., Gonzalez, C., and Pople, J.A. Gaussian 03, Revision C.02, Gaussian, Inc., Wallingford, CT, 2004.
Ray, J., Panja, N., Nandi, P.K., Martin, J.J., and Jones, W.E., J. Mol. Struct., 2008, vol. 874, nos. 1–3, pp. 121–127.
Al-Sehemi, A.G. and El-Gogary, T.M., J. Mol. Struct.: Theochem, 2009, vol. 907, nos. 1–3, pp. 66–73.
Shahwar, D., Tahir, M.N., Raza, M.A., Iqbal, B., and Naz, S., Acta Cryst., 2009, vol. 65, no. 11, p. o2637.
Barreiro, E., Casas, J.S., Couce, M.D., Sánchez, A., Sordo, J., Varela, J.M., and Vázquez-López, E.M., Cryst. Growth & Design., 2007, vol. 7, no. 10, pp. 1964–1973.
Tahmassebi, D., J. Mol. Struct.: Theochem., 2003, vol. 638, nos. 1–3, pp. 11–20.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © G.V. Baryshnikov, B.F. Minaev, V.A. Minaeva, 2011, published in Zhurnal Obshchei Khimii, 2011, Vol. 81, No. 3, pp. 481–490.
Rights and permissions
About this article
Cite this article
Baryshnikov, G.V., Minaev, B.F. & Minaeva, V.A. Theoretical study of the models of Ca2+ and Mg2+ ions binding by the methylidene rhodanine neutral and anionic forms. Russ J Gen Chem 81, 576–585 (2011). https://doi.org/10.1134/S1070363211030248
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070363211030248