Abstract
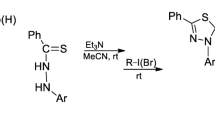
2-Aryl- and 2-furyl-4-carboxy-1,3-thiazolidines were synthesized. Their spectral properties were studied, and conformational analysis was performed. It was shown that they exist in solution as an equilibrium of neutral and zwitter-ion forms. The influence of the nature of substitutents and of their location in a benzene ring of thiazolidines as ligands of rhodium complexes on acetophenone hydrosilylation with diphenylsilane was examined. Thiazolidines containing donor substituents in the para-position of the benzene ring were found to be the most effective; maximal asymmetrical induction (55% ee) was reached in the presence of 2-(4-methoxyphenyl)-4-carboxy-1,3-thiazolidine.
Similar content being viewed by others
References
Brunner, H., Riepl, G., and Weitzer, H., Angew. Chem. Int. Ed., 1983, vol. 22, no. 4, p. 331.
Brunner, H., Becker, R., and Riepl, G., Organometallics, 1984, vol. 3, no. 9, p. 1354.
Brunner, H. and Kürzinger, A., J. Organomet. Chem., 1988, vol. 346, no. 3, p. 413.
Lipshutz, B.H., Lower, A., and Noson, K., Org. Lett., 2002, vol. 4, no. 23, p. 4045.
Lipshutz, B.H., Lower, A., Kucejko, R.J., and Noson, K., Org. Lett., 2006, vol. 8, no. 14, p. 2969.
Comte, V., Balan, C., Le Gendre, P., and Moïse, C., Chem. Comm., 2007, no. 7, p. 713.
Riemcshneider, R. and Hoyer, G.-A., Z. Naturforschg. B, 1962, vol. 17, nos. 11–12, p. 765.
Vos, O., Budke, L., Fatome, M., and Hooidonk, C., Int. J. Radiat. Biol., 1981, vol. 39, no. 3, p. 291.
Nagasawa, H., Goon, D., Muldoon, W., and Zera, R., J. Med. Chem., 1984, vol. 27, no. 5, p. 591.
Roberts, J., Amino Acids, 1995, vol. 8, no. 2, p. 113.
Gyoergydeak, Z., Kajtar-Peredy, M., Kajtar, J., and Kajtar, M., Liebigs Ann. Chem., 1987, no. 11, p. 927.
Braibante, M.E.F., Braibante, H.S., and Costenaro, E.R., Synthesis, 1999, no. 6, p. 943.
Soloway, H., Kipnis, F, Ornfelt, J., and Spoerri, P.E., J. Am. Chem. Soc., 1948, vol. 70, no. 4, p. 1667.
Brunner, H. and Obermann, U., Chem. Ber., 1989, vol. 122, no. 3, p. 499.
Gunther, H., Vvedenie v kurs spektroskopii YaMR (Introduction to NMR Spectroscopy), Moscow: Mir, 1984.
Parthasarathy, R., Paul, B., and Korytnyk, W., J. Am. Chem. Soc., 1976, vol. 98, no. 21, p. 6634.
Braga, A.L., Appelt, H.R., Schneider, P.H., Rodrigues, O.E.D., Silveira, C.C., and Wessjohann, L.A., Tetrahedron, 2001, vol. 57, no. 16, p. 3291.
Ojima, I., Li, Z., and Zhu, J., The Chemistry of Organic Silicon Compounds, Rappaport, Z. and Apeloig, Y., Eds., New York: Wiley, 1998, vol. 2, part 2, p. 1687.
Perspektivy gidrosililirovania (Hydrosilylation Prospects), Lukevits, E., Ed., Riga: Inst. Org. Sinteza Latv. Akad. Nauk, 1992.
Marciniec, B., Maciejewski, H., Pietraszuk, C., and Pawluć, P., Advances in Silicon Science, Marciniec, B., Ed., Springer, 2009, vol. 1.
Uvarov, V.M., de Vekki, D.A., Reshetilovskii, V.P., and Skvortsov, N.K., Zh. Obshch. Khim., 2010, vol. 80, no. 1, p. 39.
Brunner, H. and Obermann, U., Chem. Ber., 1989, vol. 122, no. 3, p. 499.
Gemperline, P., Practical Guide to Chemometrics, New York: Taylor and Francis, 2006.
Takeuchi, Y., Noriaki, I., Satoh, T., Koizumi, T., and Yamaguchir, K., J. Org. Chem., 1993, vol. 58, no. 7, p. 1812.
Rukovodstvo po neorganicheskomu sintezu (Inorganic Synthesis Guidelines), Brauer, E., Ed., Moscow: Mir, 1986, vol. 6, p. 1867.
Braga, A.L., Appelt, H.R., Schneider, P.H., Silveira, C.C., and Wessjohann, L.A., Tetrahedron: Asymmetry, 1999, vol. 10, no. 9, p. 1733.
Takeuchi, Y., Noriaki, I., Satoh, T., Koizumi, T., and Yamaguchir, K., J. Org. Chem., 1993, vol. 58, no. 7, p. 1812.
Laszlo, S., Liebigs Ann. Chem., 1985, no. 4, p. 657.
Oya, M., Ito, S., Harada, K.-I., Suzuki, M., and Tatematsu, A., Chemical & Pharmaceutical Bulletin, 1982, vol. 30, no. 2, p. 2705.
Szilagyi, L. and Gyorgydeak, Z., J. Am. Chem. Soc., 1979, vol. 101, no. 2, p. 427.
Howard-Lock, H.E., Lock, C. J. L., and Martins, M.L., Can. J. Chem., 1991, vol. 69, no. 11, p. 1721.
Nagasawa, H.T., Goon, D.J.W., Muldoon, W.P., and Zera, R.T., J. Med. Chem., 1984, vol. 27, no. 5, p. 591.
Confalone, P.N., Pizzolato, G., Baggiolini, E.G., Lollar, D., and Uskokovic, M.R., J. Am. Chem. Soc., 1977, vol. 99, no. 21, p. 7020.
Sutcliffe, O.B., Storr, R.C., Gilchrist, T.L., and Rafferty, P., J. Chem. Soc., Perkin Trans. 1, 2001, no. 15, p. 1795.
Schmolka, I.R. and Spoerri, P.E., J. Org. Chem., 1957, vol. 22, no. 8, p. 943.
Terol, A., Fernandez, J.-P., Robbe, Y., Chapat, J.-P., Granger, R., and Sentenac-Roumanou, H., Eur. J. Med. Chem., 1978, vol. 13, no. 2, p. 153.
Kasida, Y. and Yakugaku, Z., C.A., 1950, vol. 44, nos. 4–6, p. 1490a.
Gududuru, V., Hurh, E., Dalton, J. T., and Miller, D.D., J. Med. Chem., 2005, vol. 48, no. 8, p. 2584.
Author information
Authors and Affiliations
Additional information
Original Russian Text © A.N. Skvortsov, V.M. Uvarov, D.A. de Vekki, E.P. Studentsov, N.K. Skvortsov, 2010, published in Zhurnal Obshchei Khimii, 2010, Vol. 80, No. 10, pp. 1697–1711.
Rights and permissions
About this article
Cite this article
Skvortsov, A.N., Uvarov, V.M., de Vekki, D.A. et al. Conformational analysis, spectral and catalytic properties of 1,3-thiazolidines, ligands for acetophenone hydrosilylation with diphenylsilane. Russ J Gen Chem 80, 2007–2021 (2010). https://doi.org/10.1134/S107036321010021X
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S107036321010021X