Abstract
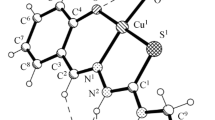
2-[(2-Hydroxyphenylimino)methyl]phenol (H2L1) and 1-[(2-hydroxyphenylimino)methyl]naphthalen-2-ol (H2L2) reacted with copper(II) acetate hydrate and sulfanilamide (Sf1), sulfathiazole (Sf2), sulfaethidole (Sf3), sulfadiazine (Sf4), and sulfadimidine (Sf5) in ethanol to give mixed-ligand copper chelates with the composition Cu(Sf1–5)(L1–2) · n H2O (n = 1, 2). All these complexes are monomeric. Salicylaldehyde imines (H2L1 and H2L2) behave as doubly deprotonated tridentate O,N,O ligands, whereas sulfanilamides (Sf1–5) are unidentate ligands. Thermolysis of the synthesized complexes includes dehydration at 70–90°C, followed by complete thermal decomposition (290–380°C). The complexes [Cu(Sf1)(L1)] · 2H2O and [Cu(Sf3)(L1)] · H2O at a concentration of 10−4 M inhibited growth and reproduction of 100% of human myeloid leukemia cells (HL-60). The inhibitory effect was 90 and 75%, respectively, at a concentration of 10−5 M, whereas no antitumor activity was observed at a concentration of 10−6 M.
Similar content being viewed by others
References
Kogan, V.A., Zelentsov, V.V., Osipov, O.A., and Burlov, A.S., Usp. Khim., 1979, vol. 48, no. 7, p. 1205.
Kasumov, V.T., Medzhidov, A.A., and Ismailov, R.G., Koord. Khim., 1986, vol. 12, no. 12, p. 1616.
Potapov, V.M., Panova, G.V., Pekshueva, E.G., and Garbar, A.V., Zh. Obshch. Khim., 1983, vol. 53, no. 7, p. 1620.
Panova, G.V., Pekshueva, E.G., Potapov, V.M., and Ashkinadze, L.D., Zh. Obshch. Khim., 1981, vol. 51, no. 6, p. 1209.
Samus’, N.M., Shlyakhov, E.N., Velishko, N.G., Burdenko, T.A., Chaika, T.S., Tsapkov, V.I., Bodyu, V.G., and Borozenets, S.P., Khim.-Farm. Zh., 1989, vol. 23, no. 9, p. 1098.
Horowitz, H.H. and Metzger, G.A., Anal. Chem., 1963, vol. 35, no. 10, p. 1464.
Topor, N.D., Vestn. Mosk. Gos. Univ., Geol., 1967, no. 1, p. 84.
Kukushkin, Yu.N., Budanova, V.F., and Sedova, G.N., Termicheskie prevrashcheniya koordinatsionnykh soedinenii v tverdoi faze (Thermal Transformations of Coordination Compounds in the Solid Phase), Leningrad: Leningr. Gos. Univ., 1981.
Kukushkin, Yu.N., Khodzhaev, O.F., Budanova, V.F., and Parpiev, N.A., Termoliz koordinatsionnykh soedinenii (Thermolysis of Coordination Compounds), Tashkent: Fan, 1986.
Afrasiabi, Z., Sinn, E., and Padhye, S., J. Inorg. Biochem., 2003, vol. 95, no. 4, p. 306.
Chumakov, Yu.M., Tsapkov, V.I., Zhanno, E., Bairak, N.N., Bochelli, G., Puar’e, D., Rua, Zh., and Gulya, A.P., Kristallografiya, 2008, vol. 53, no. 5, p. 833.
Gulea, A., Poirier, D., Roy, J., Stavila, V., Bulimestru, I., Tapcov, V., Birca, M., and Popovschi, L., J. Enzyme Inhibit. Med. Chem., 2008, vol. 23, no. 6, p. 806.
Gulya, A.P., Puar’e, D., Rua, Zh., Stavile, V.G., and Tsapkov, V.I., Moldavian Patent no. 2786, 2005; Byull. Izobret. Mold., BOPI no. 6/2005, p. 22.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © A.P. Gulya, V.I. Tsapkov, D. Poirier, K. Aruksandei, E. Pakhontsu, 2010, published in Zhurnal Obshchei Khimii, 2010, Vol. 80, No. 7, pp. 1185–1188.
Rights and permissions
About this article
Cite this article
Gulya, A.P., Tsapkov, V.I., Poirier, D. et al. Sulfanilamide copper(II) chelates with 2-[(2-hydroxyphenyl-imino)methyl]phenolom and 1-[(2-hydroxyphenylimino)-methyl]naphthalen-2-ol. Russ J Gen Chem 80, 1351–1354 (2010). https://doi.org/10.1134/S1070363210070224
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070363210070224