Abstract
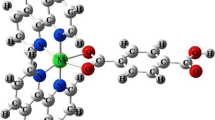
1,10-Phenanthroline, 2,2′-bi-1,10-phenanthroline, and its dianion were examined by DFT B3LYP calculations in the 6-31G** basis set using the GAMESS-2006 program complex. The rigid tetradentate heterocycle, 2,2′-bi-1,10-phenanthroline, in the reduced electron-excessive form L2− creates more favorable geometric and electronic conditions for insertion of complexing ions in its cavity, compared to the neutral ligand.
Similar content being viewed by others
References
Sammes, P.G. and Yahioglu, G., Chem. Soc. Rev., 1994, vol. 23, no. 5, p. 327.
Monk, R., Wright, L., Dungey, K., and Burke, A., in Southeast Regional Meet. of the American Chemical Society, 2002, June, p. 55.
Moiseev, I.I., Vargaftik, M.N., Gekhman, A.E., Stromnova, T.A., and Moiseeva, N.I., in Teoreticheskaya i prikladnaya neorganicheskaya khimiya (Theoretical and Applied Inorganic Chemistry), Moscow: Nauka, 1999, p. 74.
Volokitin, Y., Sinzig, J., Jongh, L.J. de, Schmid, G., Vargaftik, N., and Moiseev, I.I., Nature, 1996, vol. 384, no. 12, p. 621.
Misra Sudhindra, N. and Singh, Megh., J. Indian Chem. Soc., 1983, vol. 60, no. 2, p. 115.
Catellani, M. and Chiusoli, G.P., J. Organomet. Chem., 1988, vol. 346, no. 1, p. C27.
Beer, R.H., Jimenez, J., and Drago, R.S., J. Org. Chem., 1993, vol. 58, no. 7, p. 1746.
Shulman, A. and White, D.O., Chem.-Biol. Interact., 1973, vol. 6, no. 2, p. 407.
Ward, S.G., Taylor, R.C., Crowe, A.J., Balzarini, J., and De Clercq, E., Appl. Organomet. Chem., 1989, vol. 3, no. 5, p. 431.
Hu, C., Wang, S., Zhao, S., Shen, W., and Wang, P., Zhongguo Yaowu Huaxue Zazhi, 1994, vol. 4, no. 1, p. 32.
Pallenberg, A.J., Branca, A., Marschner, T.M., and Patt, L.M., PCT Int. Appl. WO 9427594, December 8, 1994; US Appl. 71440, June 2, 1993.
Kostova, I., Recent Pat. Anti-Cancer Drug Discov., 2006. vol. 1, no. 1, p. 1.
Pitie, M., Croisy, A., Carrez, D., Boldron, C., and Meunier, B., Chembiochem., 2005, vol. 6, no. 4, p. 686.
Powell, R.D., US Patent 5728590, March 17, 1998.
Lu, W., Vicic, D.A., and Barton, J.K., Inorg. Chem., 2005, vol. 44, no. 22, p. 7970.
Cusumano, M., Di Pietro, M.L., and Giannetto, A., Inorg. Chem., 2006, vol. 45, no. 1, p. 230.
Dupureur, C.M. and Barton, J.K., Inorg. Chem., 1997, vol. 36, no. 1, p. 33.
Barton, J.K., Pure Appl. Chem., 1989, vol. 61, no. 3, p. 563.
Jackson, B.A. and Barton, J.K., J. Am. Chem. Soc., 1997, vol. 119, no. 52, p. 12986.
Nielsen, M.B., Lomholt, H., and Becher, J., Chem. Soc. Rev., 2000, vol. 29, no. 3, p. 153.
Sun, L.X., Okada, T., Collin, J.P., and Sugihara, H., Anal. Chim. Acta, 1996, vol. 329, no. 1, p. 57.
Chi-Ying Hung, Tie-Lin Wang, Zhiqiang Shi, and Thummel, R.P., Tetrahedron, 1994, vol. 50, no. 36, p. 10685.
Hirai, M., Shinozuka, K., Sawai, H., and Ogawa, S., Chem. Lett., 1992, no. 10, p. 2023.
Rice, C.R. and Anderson, K.M., Polyhedron, 2000, vol. 19, no. 4, p. 495.
Griffiths, P.M., Loiseau, F., Puntoriero, F., Serroni, S., and Campagna, S., J. Chem. Soc., Chem. Commun., 2000, no. 10, p. 2297.
Hu Yi-Zhen, Xiang Quin, and Thummel, R.P., Inorg. Chem., 2002, vol. 41, no. 13, p. 3423.
Demidov, V.N., Kukushkin, Yu.N., Iretskii, A.V., Zhidkova, O.B., and Vedeneeva, L.N., Zh. Obshch. Khim., 1989, vol. 59, no. 8, p. 1886.
Demidov, V.N., Denisov, I.A., Belyaev, A.N., Yakovlev, V.N., and Kukushkin, Yu.N., Koord. Khim., 1991, vol. 17, no. 12, p. 1717.
Demidov, V.N., Puzenko, V.G., Savinova, A.I., Vedeneeva, L.N., and Simanova, S.A., Abstracts of Papers, XVIII Mezhdunarodnaya Chernyaevskaya konferentsiya po khimii, analitike n tekhnologii platinuvykh metallov (XVIII Int. Chernyaev Conf. on Chemistry, Analysis, and Technology of Platinum Metals), Moscow, 2006, p. 38.
Hay, P.J. and Wadt, W.R., J. Chem. Phys., 1985, vol. 82, no. 1, p. 284.
Schmidt, M.W., Baldridge, K.K., Boatz, J.A., Elbert, S.T., Gordon, S., Jensen, J.H., Koseki, S., Matsunaga, N., Nguen, K.A., Su, S.J., Windus, T.L., Dupuis, M., and Montgomery, J.A., J. Comput. Chem., 1993, vol. 14, no. 6, p. 1347.
Grigg, E.C.M., Hall, J.R., and Plowman, R.A., Aust. J. Chem., 1962, vol. 15, no. 3, p. 425.
Thornton, D.A. and Watkins, G.M., Spectrochim. Acta (A), 1991, vol. 47, no. 8, p. 1085.
Inskeep, R.G., J. Inorg. Nucl. Chem., 1962, vol. 24, no. 5, p. 763.
Howell, S.L. and Gordon, K.C., J. Phys. Chem. A, 2004, vol. 108, no. 13, p. 2536.
Denisova, A.S. and Lysinova, M.B., Zh. Obshch. Khim., 2002, vol. 72, no. 6, p. 881.
Rosenberger, H., Pettig, M., and Madeja, K., Z. Chem., 1966, vol. 6, no. 1, p. 30.
Baranovskii, V.I. and Lyubimova, O.O., Elektron. Zh. “Issled. Ross.,” 2001, http://zhurnal.ape.relarn.ru/articles/2001/146.pdf , p. 1682.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © N.S. Panina, V.N. Demidov, S.A. Simanova, 2008, published in Zhurnal Obshchei Khimii, 2008, Vol. 78, No. 5, pp. 771–776.
Rights and permissions
About this article
Cite this article
Panina, N.S., Demidov, V.N. & Simanova, S.A. A DFT study of 2,2′-bi-1,10-phenanthroline and its reduced form as a potential ligand for new tetraaza chromophore complexes. Russ J Gen Chem 78, 913–918 (2008). https://doi.org/10.1134/S1070363208050137
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070363208050137