Abstract
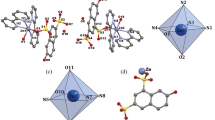
A series of anionic phenylsilicon(IV) bis-catecholate complexes, where (Cat1)– = catecholate, (Cat2)– = 3,4,5,6-tetrabromocatecholate, (Cat3)– = 4-cyanocatecholate, (Cat4)– = 4-nitrocatecholate, (Cat5)– = 3-fluorocatecholate, and (Cat6)– = 4,5-dibromocatecholate, were prepared by the reaction of trimethoxyphenylsilane with two equivalents of catechol or its derivatives containing electron-withdrawing substituents in the benzene ring in the presence of dicyclohexylamine. The complexes were characterized by IR spectra and 1H, 13C, and 29Si NMR spectra, voltammetry, and ESR. The heteroligand anionic phenylsilicon(IV) complexes crystallized from solutions as salts (Chex2NH2)[PhSi(Cat1)2] (I), (Chex2NH2)-[PhSi(Cat2)2]·0.5C6H14 (II), (Chex2NH2)[PhSi(Cat3)2]·2C6H6 (III), (Chex2NH2)[PhSi(Cat4)2]· H2O (IV), (Chex2NH2)[PhSi(Cat5)2] (V), and (Chex2NH2)[PhSi(Cat6)2] (VI) (Chex2\({\text{NH}}_{2}^{ + }\) = dicyclohexylammonium). The composition and structure of the products were confirmed by X-ray diffraction data (CCDC nos. 2150293–2150297 for I–V, respectively). The effect of substituent in the benzene ring on the electronic structure of the anion was studied by PBE0/6-311G(d,p) quantum chemical calculations with allowance for non-specific solvation (PCM model). The oxidation potentials of II–VI were measured; radical formation upon electrochemical oxidation and reduction was observed for II.









Similar content being viewed by others
REFERENCES
Corce, V., Chamoreau, L.-M., Derat, E., et al., Angew. Chem., Int. Ed. Engl., 2015, vol. 54, no. 39, p. 11414. https://doi.org/10.1002/anie.201504963
Raynor, K.D., May, G.D., Bandarage, U.K., et al., Org. Chem., 2018, vol. 83, no. 3, p. 1551. https://doi.org/10.1021/acs.joc.7b02680
Lin, K., Wiles, R.J., Kelly, C.B., et al., ACS Catal., 2017, vol. 7, no. 8, p. 5129. https://doi.org/10.1021/acscatal.7b01773
Levernier, E., Corcé, V., Rakotoarison, L.-M., et al., Org. Chem. Front., 2019, vol. 6, no. 9, p. 1378. https://doi.org/10.1039/C9QO00092E
Lévêque, C., Chenneberg, L., Corcé, V., et al., Org. Chem. Front., 2016, vol. 3, no. 4, p. 462. https://doi.org/10.1039/C6QO00014B
Patel, N.R., Kelly, C.B., Siegenfeld, A.P., et al., ACS Catal., 2017, vol. 7, no. 3, p. 1766. https://doi.org/10.1021/acscatal.6b03665
Cartier, A., Levernier, E., Corcé, V., et al., Angew. Chem., 2019, vol. 131, no. 6, p. 1803. https://doi.org/10.1002/ange.201811858
Levernier, E., Jaouadi, K., Zhang, H.-R., et al., Chem.-Eur. J., 2021, vol. 27, no. 34, p. 8782. https://doi.org/10.1002/chem.202100453
Blessing, R.H., Acta Crystallogr., Sect. A: Found. Crystallogr., 1995, vol. A51, no. 1, p. 33. https://doi.org/10.1107/S0108767394005726
APEX3 Suite for Crystallographic Software — Single Crystal X-ray Diffraction, Bruker AXS, 2014.
Dolomanov, O.V., Bourhis, L.J., Gildea, R.J., et al., J. Appl. Crystallogr., 2009, vol. 42, no. 2, p. 339. https://doi.org/10.1107/S0021889808042726
Sheldrick, G.M., Acta Crystallogr., Sect. A: Cryst. Adv., 2015, vol. 71, no. 1, p. 3. https://doi.org/10.1107/S2053273314026370
Sheldrick, G.M., Acta Crystallogr., Sect. C: Struct. Chem., 2015, vol. 71, no. 1, p. 3. https://doi.org/10.1107/S2053229614024218
Krylov, A.I. and Gill, P.M.W., WIREs Comput. Mol. Sci., 2013, vol. 3, no. 3, p. 317. https://doi.org/10.1002/wcms.1122
Epifanovsky, E., Gilbert, A.T.B., Feng, X., et al., J. Chem. Phys., 2021, vol. 155, no. 8, p. 084801. https://doi.org/10.1063/5.0055522
Neugebauer, H., Bohle, F., Bursch, M., et al., J. Phys. Chem. A, 2020, vol. 124, no. 35, p. 7166. https://doi.org/10.1021/acs.jpca.0c05052
Lu, T. and Chen, F., J. Comput. Chem., 2012, vol. 33, no. 5, p. 580. https://doi.org/10.1002/jcc.22885
Humphrey, W., Dalke, A., and Schulten, K., J. Mol. Graph., 1996, vol. 14, no. 1, p. 33. https://doi.org/10.1016/0263-7855(96)00018-5
Boer, F.P., Flynn, J.J., and Turley, J.W., J. Am. Chem. Soc., 1968, vol. 90, no. 25, p. 6973. https://doi.org/10.1021/ja01027a014
Holmes, R.R., Day, R.O., Chandrasekhar, V., et al., Inorg. Chem., 1985, vol. 24, no. 13, p. 2009. https://doi.org/10.1021/ic00207a012
Holmes, R.R., Day, R.O., Harland, J.J., et al., Organometallics, 1984, vol. 3, no. 3, p. 341. https://doi.org/10.1021/om00081a001
Holmes, R.R. and Deiters, J.A., J. Am. Chem. Soc., 1977, vol. 99, no. 10, p. 3318. https://doi.org/10.1021/ja00452a021
Korlyukov, A.A., Usp. Khim., 2015, vol. 84, no. 4, p. 422.
Kramarova, E.P., Volodin, A.D., Negrebetsky, V.V., et al., Molecules, 2021, vol. 26, no. 12, p. 3548. https://doi.org/10.3390/molecules26123548
Korlyukov, A.A., Shipov, A.G., Kramarova, E.P., et al., Izv. Akad. Nauk. Ser. Khim., 2008, no. 10, p. 2055.
O’Keeffe, M., Peskov, M.A., Ramsden, S.J., et al., Acc. Chem. Res., 2008, vol. 41, no. 12, p. 1782. https://doi.org/10.1021/ar800124u
Politzer, P. and Murray, J.S., Theoretical and Computational Chemistry, Chapter 8: The Averag Local Ionization Energy: Concepts and Applications, Toro-Labbe, A., Ed., 2007, vol. 19, p. 119. https://doi.org/10.1016/S1380-7323(07)80009-4
Namazian, M., Lin, C.Y., and Coote, M.L., J. Chem. Theory Comput., 2010, vol. 6, no. 9, p. 2721. https://doi.org/10.1021/ct1003252
ACKNOWLEDGMENTS
Elemental analysis, X-ray diffraction studies, and recording of NMR and IR spectra were supported by the Ministry of Science and Higher Education of the Russian Federation and performed using research equipment of the Molecular Structure Investigation Center of the Nesmeyanov Institute of Organoelement Compounds, Russian Academy of Sciences. P.A. Buikin and A.A. Korlyukov are grateful to the Interdepartmental Supercomputer Center, Russian Academy of Sciences, for providing access to computational resources and software.
Funding
This study was supported by the Russian Foundation for Basic Research (grant no. 19-29-08021).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Dedicated to Academician Yu.A. Zolotov in the year of his 90th birthday
Translated by Z. Svitanko
Supplementary Information
Rights and permissions
About this article
Cite this article
Kramarova, E.P., Negrebetskii, V.V., Volodin, A.D. et al. Synthesis, Crystal Structure, and Properties of Phenylsilicon(IV) Bis-catecholate Complexes. Russ J Coord Chem 48, 647–658 (2022). https://doi.org/10.1134/S1070328422090019
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070328422090019