Abstract
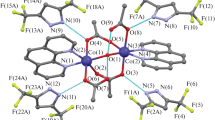
The reaction of aqueous copper(II) acetate with phenanthroline (Phen) gave the complex PhenCu(OOCMe)2 ∙ 2MeCN (I), which was converted to the single crystals of the monomer PhenCu(OOCMe)2 ∙ 1.5CH2Cl2 (II) upon recrystallization of dichloromethane. Recrystallization of I from wet benzene gave the single crystals of PhenCu(OOCMe)2(OH2) ∙ 0.5 C6H6 ∙ 0.5MeCN (III) containing a coordinated water molecule. The reaction of I with 3.5 dimethylpyrazole (HDmpz) (1 : 2) resulted in the synthesis of PhenCu(OOCMe)2HDmpz ∙ 0.5H2O ∙ 0.5CH2Cl2 (IV) in which the pyrazole molecule occupied the same coordination site as the water molecule in III. The reaction of I with trifluoromethanesulfonic acid yielded PhenCu(OH2)(NCMe)(Otf)2 (V), which was also prepared by the reaction of copper acetate with HOtf followed by the addition of 1 mole of Phen ∙ H2O. Monomer V reacted with 2 moles of HDmpz to be converted to PhenCu(HDmpz)2(Otf)2 (VI). Compounds I–VI were characterized by elemental analysis data, IR spectra, and X-ray diffraction (CCDC nos. 1948544 (I), 1948541 (II), 1987813 (III), 1948542 (IV), 1948540 (V), 1948543 (VI)). The geometry of complexes and the effect of solvent used for the reactions and crystallization on the molecular packing in the crystals were considered on the basis of X-ray diffraction data.







Similar content being viewed by others
REFERENCES
Porai-Koshits, M.A., Itogi Nauki Tekhn. Ser. Kristallokhimiya, Moscow: VINITI, 1981, vol. 15.
Mehrotra, R.C. and Bohra, R., Metal Carboxylates, London: Acad. Press, 1983, р. 396.
Cotton, F.A., Wilkincon, G., Murillo, C.A., and Bochmann, M., Advanced Inorganic Chemistry, New York: Wiley, 1999.
Valle, H.U., Riley, K.M., Russell, D.E., Wolgemuth, D.K., et al., Chem. Sel., 2018, vol. 3, p. 5143. https://doi.org/10.1002/slct.201800588
Stopka, T., Marzo, L., Zurro, M., and Janich, S., Angew. Chem., Int. Ed., 2015, vol. 54, p. 5049. https://doi.org/10.1002/anie.201411726
Dawsey, A.C., Li, V., Hamilton, K.C., and Williams, T.J., Dalton Trans., 2012, vol. 41, p. 7994. https://doi.org/10.1039/c2dt00025c
Comerford, J.W., Hart, S.J., North, M., and Whitwood, A.C., Cat. Sci. Tech., 2016, vol. 6, p. 4824.https://doi.org/10.1039/C6CY00134C
Thongkam, P., Jindabot, S., Prabpai, S., et al., RCS Adv., 2015, vol. 5, p. 55847. https://doi.org/10.1039/C5RA06933E
Lippard, S.J. and Berg, J.M., Principles of Bioinorganic Chemistry, Mill Valley: University Science Books, 1994.
Solomon, E.I., Sundaram, U.M., and Machonkin, T.E., Chem. Rev., 1996, vol. 96, p. 2563.https://doi.org/10.1021/cr950046o
Kaim, W. and Rall, J., Angew. Chem., Int. Ed. Engl., 1996, vol. 35, p. 43.https://doi.org/10.1002/anie.199600431
Holm, R.H., Kennepohl, P., and Solomon, E.I., Chem. Rev., 1996, vol. 96, p. 2239.https://doi.org/10.1021/cr9500390
Kim, E., Chufan, E.E., Kamaraj, K., and Karlin, K.D., Chem. Rev., 2004, vol. 104, p. 1077.
Nefedov, S.E., Russ. J. Inorg. Chem., 2006, vol. 51, Suppl. 1, p. 49.https://doi.org/10.1134/S0036023606130031
SMART (control) and SAINT (integration) Software. Version 5.0, Madison: Bruker AXS Inc., 1997.
SAINT: Area-Detector Integration Sofware, Madison: Bruker AXS Inc., 2012.
Sheldrick, G.M., SADABS. Program for Scaling and Correction of Area Detector Data, Göttingen: Univ. of Göttingen, 1997.
Sheldrick, G.M., Acta Crystallogr., Sect. C:Struct. Chem., 2015, vol. 71, p. 3. https://doi.org/10.1107/S2053229614024218
Barquin, M., Gonzalez-Garmendia, M.J., Larrinaga, E., et al., Anorg.Allg. Chem., 2005, vol. 631, p. 2151.https://doi.org/10.1002/zaac.200570045
Barquin, M., Cocera, N.G., Garmendia, M.J., and Larrinaga, L., Inorg. Chim. Acta, 2010, vol. 363, p. 127. https://doi.org/10.1016/j.ica.2009.09.034
Neto, J.A., Silva, C.C., Ribeiro, L., et al., Z. Krist. Cryst. Mater., 2019, vol. 234, p. 119.
Rice, C.R., Onions, S., and Vidal, N., Eur. J. Inorg. Chem., 2002, p. 1985.
Perlepes, S.P., Libby, E., Streib, W.E., et al., Polyhedron, 1992, vol. 11, p. 923 https://doi.org/10.1016/S0277-5387(00)83342-2
Costamagna, J., Caruso, F., Rossi, M., et al., J. Coord. Chem., 2001, vol. 54, p. 247.https://doi.org/10.1080/00958970108022638
Kaewthong, A., Sukwattanasinitt, M., and Muangsin, N., Acta Crystallogr., Sect E: Struct. Rep. Online, 2011, vol. 67, p. 712.https://doi.org/10.1107/S0108767311081980
Carlucci, L., Ciani, G., and Gramaccioli, A., CrystEngComm, 2000, vol. 2, p. 154.https://doi.org/10.1039/B006306L
Seidel, R.W., Dietz, C., and Oppel, I.M., Z. Anorg. Allg. Chem., 2011, vol. 637, p. 94.https://doi.org/10.1002/zaac.201000266
ACKNOWLEDGMENTS
X-ray diffraction studies of the complexes were performed using research equipment of the Center for Collective Use of Physicochemical Investigation Methods of the Kurnakov Institute of General and Inorganic Chemistry, Russian Academy of Sciences, functioning with the support of the State Assignment of the Kurnakov Institute of General and Inorganic Chemistry, Russian Academy of Sciences, in the field of fundamental research.
Funding
This work was performed within the framework of the State Assignment of the Kurnakov Institute of General and Inorganic Chemistry, Russian Academy of Sciences, in the field of fundamental research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by Z. Svitanko
Rights and permissions
About this article
Cite this article
Uvarova, M.A., Nefedov, S.E. Reactions of PhenCuX2 (X = OOCMe, O3SCF3) with 3,5-Dimethylpyrazole: Effect of the Anion Nature on the Composition and Structure of the Complexes. Russ J Coord Chem 46, 125–136 (2020). https://doi.org/10.1134/S1070328420020062
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070328420020062