Abstract
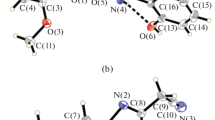
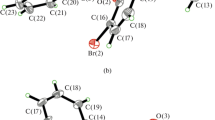
Two new oxodovanadium(V) complexes, [VO(L)(OEt)(MeOH)] (I) and [VO(L)(Bha)] · EtOH (II), where L is the anion of 2-chloro-N'-(3,5-dichloro-2-hydroxybenzylidene)benzohydrazide (H2L), Bha is the anion of 2-hydroxybenzohydroxamic acid (HBha), were prepared and characterized by IR, UV-Vis and single crystal X-ray determination (CIF files CCDC nos. 1840661 (I) and 1840662 (II)). Complex I crystallizes as the monoclinic space group P21/c with unit cell dimensions a = 8.272(1), b = 21.326(2), c = 12.979(1) Å, β = 107.173(2)°, V = 2187.5(4) Å3, Z = 4, R1 = 0.0811, wR2 = 0.2152, GOOF = 1.048. Complex II crystallizes as the triclinic space group P\(\bar {1}\) with unit cell dimensions a = 7.407(2), b = 14.195(2), c = 14.330(2) Å, α = 117.262(2)°, β = 92.947(2)°, γ = 95.771(2)°, V = 1324.4(4) Å3, Z = 2, R1 = 0.0919, wR2 = 0.1539, GOOF = 0.986. X-ray analysis indicates that the complexes are mononuclear vanadium(V) species, with the V atoms in octahedral coordination. The complexes were evaluated for their antibacterial (Bacillus subtilis, Staphylococcus aureus, Escherichia coli, and Pseudomonas fluorescence) and antifungal (Candida albicans and Aspergillus niger) activities by MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide) method. The two complexes have from medium to strong activities against B. subtilis, S. aureus, and E. coli.


Similar content being viewed by others
REFERENCES
Kaplancikli, Z.A., Altintop, M.D., Ozdemir, A., et al., Lett. Drug Des. Discov., 2014, vol. 11, no. 3, p. 355.
Narisetty, R., Chandrasekhar, K.B., Mohanty, S., et al., Lett. Drug Des. Discov., 2013, vol. 10, no. 7, p. 620.
Zhi, F., Shao, N., Wang, Q., et al., J. Struct. Chem., 2013, vol. 54, no. 1, p. 148.
Ozdemir, A., Turan-Zitouni, G., Kaplancikli, Z.A., et al., J. Enzyme Inhib. Med. Chem., 2008, vol. 23, no. 2, p. 470.
Loncle, C., Brunel, J.M., Vidal, N., et al., Eur. J. Med. Chem., 2004, vol. 39, no. 12, p. 1067.
Liu, Y.-C., Wang, H.-L., Tang, S.-F., et al., Anticancer Res., 2014, vol. 34, no. 10, p. 6034.
Krishnamoorthy, P., Sathyadevi, P., Cowley, A.H., et al., Eur. J. Med. Chem., 2011, vol. 46, no. 8, p. 3376.
Zhang, M., Xian, D.-M., Li, H.-H., et al., Aust. J. Chem., 2012, vol. 65, no. 4, p. 343.
Shi, L., Ge, H.-M., Tan, S.-H., et al., Eur. J. Med. Chem., 2007, vol. 42, no. 4, p. 558.
Rai, N.P., Narayanaswamy, V.K., Govender, T., et al., Eur. J. Med. Chem., 2010, vol. 45, no. 6, p. 2677.
Wazalwar, S.S., Bhave, N.S., Dikundwar, A.G., et al., Synth. React. Inorg. Met.-Org. Nano-Met. Chem., 2011, vol. 41, no. 5, p. 459.
Liu, J.-L., Sun, M.-H., and Ma, J.-J., Synth. React. Inorg. Met.-Org. Nano-Met. Chem., 2015, vol. 45, no. 1, p. 117.
Chohan, Z.H., Sumrra, S.H., Youssoufi, M.H., et al., Eur. J. Med. Chem., 2010, vol. 45, no. 7, p. 2739.
Taheri, O., Behzad, M., Ghaffari, A., et al., Transition Met. Chem., 2014, vol. 39, no. 2, p. 253.
SMART (version 5.625) and SAINT (version 6.01), Madison: Bruker AXS Inc., 2007.
Sheldrick, G.M., SADABS, Program for Empirical Absorption Correction of Area Detector, Göttingen: Univ. of Göttingen, 1996.
Sheldrick, G.M., SHELXTL, Version 5.1, Software Reference Manual, Madison: Bruker AXS, Inc., 1997.
Meletiadis, J., Meis, J.F.G.M., Mouton, J.W., et al., J. Clin. Microbiol., 2000, vol. 38, no. 8, p. 2949.
Sarkar, A. and Pal, S., Polyhedron, 2007, vol. 26, no. 6, p. 1205.
Monfared, H.H., Alavi, S., Bikas, R., et al., Polyhedron, 2010, vol. 29, no. 18, p. 3355.
Zhang, X.-T., Zhan, X.-P., Wu, D.-M., et al., Chin. J. Struct. Chem., 2002, vol. 21, no. 7, p. 629.
Guo, S., Ding, Y., Li, A., et al., Z. Anorg. Allg. Chem., 2018, vol. 644, no. 19, p. 1172.
FUNDING
This work was financially supported by K.C. Wong Magna Fund in Ningbo University, Ningbo natural science fund (project no. 201701HJ-B01019) and Ningbo Education Research Project (project no. 2017YZD001).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wu, S.M., Qiu, X.Y., Wang, J.C. et al. Synthesis, Characterization, and Crystal Structures of Oxidovanadium(V) Complexes Derived from 2-Chloro-N'-(3,5-dichloro-2-hydroxybenzylidene)benzohydrazide with Antimicrobial Activity. Russ J Coord Chem 45, 378–384 (2019). https://doi.org/10.1134/S1070328419040109
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070328419040109