Abstract
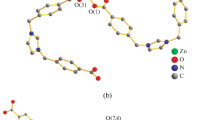
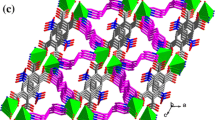
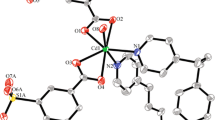
Metal-organic 1D coordination polymers of Zn(II) and Cd(II), [{Zn(3-Bphz)(H2O)4}(3-Bphz)(NO3)2]n (I), [Zn(3-Bphz)I2]n (II), [Cd(3-Bphz)I2]n (III), [Cd(4-Bphz)(CH3COO)2(H2O)]n (IV), and [Cd(4-Bphz)(NO3)2(H2O)2]n (V), containing azines of the N,N' type, 1,2-bis(pyridin-3-ylmethylene)hydrazine (3-Bphz) and 1,2-bis(pyridin-4-ylmethylene)hydrazine (4-Bphz), as bridging ligands are synthesized. The compositions and structures of the compounds are confirmed by the data of elemental analysis, IR and NMR spectroscopy, and single-crystal X-ray diffraction analysis (CIF files CCDC nos. 1812634–1812638 for I–V). Coordination polymers I–III have zigzag structures. The octahedral environment of the Zn2+ ion in compound I is formed by two 3-Bphz ligands and four water molecules. The external sphere contains nitrate anions and uncoordinated 3-Bphz molecules. In isomorphous compounds II and III, the tetrahedral environment of the metal is formed by two nitrogen atoms of two bridging 3-Bphz ligands and two iodine atoms. Coordination polymers IV and V are linear. The coordination polyhedron of the Cd2+ ion in compound IV is a pentagonal bipyramid, two vertices of which are occupied by the nitrogen atoms of two 4-Bphz molecules, and the equatorial plane is formed by two bidentate-chelating acetate anions and one water molecule. In compound V, the octahedral environment of the Сd2+ ion is formed by two molecules of the 4-Bphz ligand, two monodentate nitrate anions, and two water molecules. All complexes are weak luminophores emitting in the blue-green spectral range.







Similar content being viewed by others
REFERENCES
Janiak, C., Dalton Trans., 2003, p. 2781.
Robin, A.Y. and Fromm, K.M., Coord. Chem. Rev., 2006, vol. 250, p. 2127.
Rowsell, J.L.C. and Yaghi, O.M., Microporous Mesoporous Mater., 2004, vol. 73, p. 3.
Croitor, L., Coropceanu, E., Masunov, A., et al., J. Phys. Chem., 2014, vol. 118, p. 9217.
Coropceanu, E., Rija, A., Lozan, V., et al., Cryst. Growth Des., 2016, vol. 16, p. 814.
Croitor, L., Coropceanu, E., Chisca, D., et al., Cryst. Growth Des., 2014, vol. 14, p. 3015.
Coropceanu, E.B., Croitor, L., Wicher, B., et al., Inorg. Chim. Acta, 2009, vol. 362, p. 2151.
Croitor, L., Coropceanu, E., Petuhov, O., et al., Dalton Trans., 2015, vol. 44, p. 7896.
Chisca, D., Croitor, L., Coropceanu, E., et al., Cryst-EngComm, 2016, vol. 18, p. 384.
Chen, C.-L., Kang, B.-S., and Su, C.-Y., Aust. J. Chem., 2006, vol. 59, p. 3.
Robinson, F. and Zaworotko, M.J., Chem. Commun., 1995, p. 2413.
Sailaja, S. and Rajasekharan, M.V., Inorg. Chem., 2000, vol. 39, p. 4586.
Du, M., Bu, X.-H., Huang, Z., et al., Inorg. Chem., 2003, vol. 42, p. 552.
Gao, E.-Q., Cheng, A.-L., Xu, Y.-X., et al., Cryst. Growth Des., 2005, vol. 5, p. 1005.
MacGillivray L.R., Groeneman, R.H., and Atwood, L., J. Am. Chem. Soc., 1998, vol. 120, p. 2676.
Liu, Y.-Y., Yi, L., Ding, B., et al., Inorg. Chem. Commun., 2007, vol. 10, p. 517.
Zhang, G.-Q., Yang, G.-Q., and Ma, J.-S., Cryst. Growth Des., 2006, vol. 6, p. 1897.
Kumar, D.K., Das, A., and Dastidar, P., Cryst. Growth Des., 2006, vol. 6, p. 1903.
Huang, X.-C., Zhang, J.-P., and Chen, X.-M., Cryst. Growth Des., 2006, vol. 6, p. 1194.
Oh, M., Stern, C.L., and Mirkin, C.A., Inorg. Chem., 2005, vol. 44, p. 2647.
Ciurtin, D.M., Dong, Y.-B., Smith, M.D., et al., Inorg. Chem., 2001, vol. 40, p. 2825.
Diskin-Posner, Y., Patra, G.K., and Goldberg, I., Dalton Trans., 2001, p. 2775.
Gao, E.-Q., Cheng, A.-L., Xu, Y.-X., et al., Inorg. Chem., 2005, vol. 44, p. 8822.
Withersby, M.A., Blake, A.J., Champness, N.R., et al., Inorg. Chem., 1999, vol. 38, p. 2259.
Carlucci, L., Ciani, G., and Proserpio, D.M., J. Chem. Soc., Dalton Trans. (1972–1999), 1999, p. 1799.
Dong, Y.-B., Smith, M.D., Layland, R.C., and zur Loye, H.-C., Chem. Mater., 2000, vol. 12, p. 1156.
Yang, W. and Xiang Lin, X., Inorg. Chem., 2009, vol. 48, no. 23, p. 11067.
Granifo, J., Moreno, Y., Garland, M.T., et al., J. Mol. Struct., 2010, vol. 983, p. 76.
Jung, O.-S., Park, S.H., Kim, K.M., and Jang, H.G., Inorg. Chem., 1998, vol. 37, p. 5781.
Zaman, M.B., Smith, M.D., Ciurtin, D.M., and zur Loye, H.-C., Inorg. Chem., 2002, vol. 41, p. 4895.
Chen, C.-L., Goforth, A.M., Smith, M.D., et al., Inorg. Chem., 2005, vol. 44, p. 8762.
Patra, G.K. and Glodberg, I., Cryst. Growth Des., 2003, vol. 3, p. 321.
Chen, C.-L., Kang, B.-S., and Su, C.-Y., Aust. J. Chem., 2006, vol. 59, p. 3.
Dong, Y.-B., Layland, R.C., Smith, M.D., et al., Inorg. Chem., 1999, vol. 38, p. 3056.
Cui, Y., Ngo, H.L., and Lin, W., Chem. Commun., 2003, p. 1388.
Chen, C.-T. and Suslick, K.S., Coord. Chem. Rev., 1993, vol. 128, p. 293.
Leong, W.L. and Vittal, J.J., Chem. Rev., 2011, vol. 111, p. 688.
Song, Y., Yu, L., Gao, Y., et al., Inorg. Chem., 2017, vol. 56, p. 11603.
Miyasaka, H., Julve, M., Yamashita, M., and Clérac, R., Inorg. Chem., 2009, vol. 48, p. 3420.
Mahmoudi, G., Gurbanov, A.V., Rodríguez-Hermida, S., et al., Inorg. Chem., 2017, vol. 56, p. 9698.
Kennedy, A.R. and Waterson, F.R.N., Acta Crystallogr. Sect. C: Cryst. Struct. Commun., 2003, vol. 59, p. o613.
Calahorro, A.J., San Sebastian, E., Salinas-Castil-lo, A., et al., CrystEngComm, 2015, vol. 17, p. 3659.
Manna, B., Singh, S., and Ghosh, S.K., J. Chem. Sci., 2014, vol. 126, no. 5, p. 1417.
Croitor, L., Coropceanu, E.B., Siminel, A.V., et al., Cryst. Growth Des., 2011, vol. 11, p. 3536.
Croitor, L., Coropceanu, E.B., Masunov, A.E., et al., Cryst. Growth Des., 2014, vol. 14, p. 3935.
Croitor, L., Coropceanu, E.B., Duca, G., et al., Polyhedron, 2017, vol. 129, p. 9.
Sheldrick, G.M., Acta Crystallogr., Sect. A: Found. Crystallogr., 2008, vol. 64, p. 112.
Paulin, S., Kelly, P., Williams, K.B., et al., Acta Crystallogr., Sect. E: Struct. Rep. Online, 2007, vol. 63, р. m420.
Wang, Q., Liang, B., Zhang, J.-Y., et al., Z. Anorg. Allg. Chem., 2007, vol. 633, p. 2463.
Mahmoudi, G., Morsali, A., Hunter, A.D., and Zeller, M., CrystEngComm, 2007, vol. 9, p. 704.
Metelitsa, A.V., Burlov, A.S., and Borodkina, I.G., et al., Russ. J. Coord. Chem., 2006, vol. 32, no. 12, p. 858. doi 10.1134/S1070328406120025
Author information
Authors and Affiliations
Corresponding authors
Additional information
Translated by E. Yablonskaya
Rights and permissions
About this article
Cite this article
Lozovan, V., Coropceanu, E.B., Bourosh, P.N. et al. Coordination Polymers of Zn and Cd Based on Two Isomeric Azine Ligands: Synthesis, Crystal Structures, and Luminescence Properties. Russ J Coord Chem 45, 11–21 (2019). https://doi.org/10.1134/S107032841901007X
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S107032841901007X