Abstract
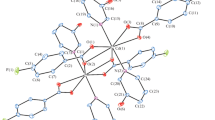
The coordination polymers (CPs) {[Cd(Pydc)(H2O)3] · PydcH2} (I) and [Mn(Pydc)(H2O)3] · PydcH2} (II) were obtained by the reaction of CdSO4 · 5H2O or MnCl2 · 4H2O with pyridine-2,6-dicarboxylic acid (PydcH2). The structures of the CPs I and II were characterized by IR, UV-Vis, TGA, and X-ray single crystal analysis (CIF files CCDC nos. 1417757 (I), 1417758 (II)). The network structures of I and II are constructed by an infinite number of discrete binuclear molecules and free PydcH2. The structures of the CPs I and II connected by the extensive H-bonds and π–π stacking, forming a 3D-network. The CPs I and II were screened to test their antimicrobial activities against different species of bacteria and fungi.
Similar content being viewed by others
References
Yaghi, O.M., O’Keeffe, M., Ockwig, N.W., et al., Nature, 2003, vol. 423, p. 705.
Coronado, E. and Espallargas, G.M., Chem. Soc. Rev., 2013, vol. 42, p. 1525.
Pu, L., Acc. Chem. Res., 2012, vol. 45, p. 150.
Bodnarchuk, M.I., Erni, R., Krumeich, F., and Kovalenko, M.V., Nano Lett., 2013, vol. 13, p. 1699.
Suh, M.P., Park, H.J., Prasad, T.K., and Lim, D.W., Chem. Rev., 2012, vol. 112, p. 782.
Duriska, M.B., Neville, S.M., Lu, J.-Z., et al., Angew. Chem., Int. Ed., 2009, vol. 48, p. 8919.
Lu, Z.-Z., Zhang, R., Li, Y.-Z., et al., J. Am. Chem. Soc., 2011, vol. 133, p. 4172.
Etaiw, S.E.H. and El-Bendary, M.M., Appl. Catal., B, 2012, vol. 126, p. 326.
Etaiw, S.E.H., Sultan, A.S., and El-Bendary, M.M., J. Organomet. Chem., 2011, vol. 696, p. 1668.
Etaiw, S.E.H. and El-Bendary, M.M., Polyhedron, 2015, vol. 87, p. 383.
Etaiw, S.E.H. and El-Bendary, M.M., Inorg. Chim. Acta, 2015, vol. 435, p. 167.
He, Y.P., Tan, Y.X., and Zhang, J., Cryst. Growth Des., 2014, vol. 14, p. 3493.
Yang, Q.X., Chen, X.Q., Chen, Z.J.Y., et al., Chem. Commun., 2012, vol. 48, p. 10016.
Barman, S., Khutia, A., Koitz, R., et al., J. Mater. Chem. A, 2014, vol. 2, p. 18823.
Wu, H., Yang, J., Su, Z.M., et al., J. Am. Chem. Soc., 2011, vol. 133, p. 11406.
Hu, J.S., Huang, L.F., Yao, X.Q., et al., Inorg. Chem., 2011, vol. 50, p. 2404.
Miao, H., Wang, Y., Cui, Y.M., and Liu, Z.D., Z. Anorg. Allg. Chem., 2014, vol. 640, p. 1514.
Cai, Y.-P._Chen, C.-L., et al., J. Am. Chem. Soc., 2003, vol. 125, p. 8595.
Song, X.-Z., Song, S.-Y., Qin, C., et al., Cryst. Growth Des., 2012, vol. 12, p. 253.
Munakata, M., Wu, L.P., et al., Inorg. Chem., 1997, vol. 36, p. 5416.
Kajiwara, T. and Ito, T., J. Chem. Soc., Dalton Trans., 1998, p. 3351.
Kamiyama, A., Noguchi, T., Kajiwara, T., and Ito, T., Angew. Chem., Int. Ed., 2000, vol. 39 p, p. 3130.
Okabe, N. and Oya, N., Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 2000, vol. 56, p. 1416.
Odoka, M., Kusano, A., and Okabe, N., Acta Crystallogr., Sect. E: Struct. Rep. Online, 2002, vol. 58, p. m25.
French, F.A., Blanz, E.J., Amaral, J.R.D., and French, D.A., J. Med. Chem., 1970, vol. 13, p. 11179.
Serbest, K., Özen, A., Unver, A.Y., et al., J. Mol. Struct., 2009, vol. 922, p. 1.
Powell, J.F., Biochem. J., 1953, vol. 54, p. 205.
Church, B.S. and Halvorson, H., Nature, 1959, vol. 183, p. 124.
Chung, L., Rajan, K.S., Merdinger, E., and Grecz, N., Biophys. J., 1971, vol. 11, p. 469.
Drew, M.G.B., Mattews, R.W., and Walton, R.A., J. Chem. Soc. A, 1970, p. 1405.
Schwarzenbach, D., Inorg. Chem., 1970, vol. 9 p, 2391.
Palmer, K.J., Wong, R.Y., and Lewis, J., Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem., 1972, vol. 28, p. 223.
Quaglieri, P.P., Loiseleur, H., and Thomas, G., Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem., 1972, vol. 28, p. 2583.
Laine, Â.P., Gourdon, A., and Launay, J.-P., Inorg. Chem., 1995, vol. 34, p. 5129.
Okabe, N. and Oya, N., Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 2000, vol. 56, p. 305.
Laine, Â.P., Strahs, A.G., and Dickerson, R.E., Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem., 1968, vol. 24, p. 571.
Gourdon, A., Launay, J.-P., and Tuchagues, J.-P., Inorg. Chem., 1995, vol. 34, p. 5150.
Aghabozorg, H., Sadr-Khanlou, E., Shokrollahi, A., et al., J. Iran. Chem. Soc., 2009, vol. 6, p. 55.
Aghajani, Z., Aghabozorg, H., Sadr-Khanlou, E., et al., J. Iran. Chem. Soc., 2009, vol. 6, p. 373.
Deacon, G.B. and Philips, R.J., Coord. Chem. Rev., 1980, vol. 33, p. 227.
Valeur, B., Molecular Fluorescence Principles and Applications, Weinheim: Wiley-VCH, 2002, p. 59.
Jaffé, H.H. and Orchin, M., Theory and Applications of Ultraviolet Spectroscopy, Wiley, 1970.
Efthimiadou, E.K., Psomas, G., et al., J. Inorg. Biochem., 2007, vol. 101, p. 525.
Lawrence, P.G., Harold, P.L., and Francis, O.G., Antibiot. Chemother., 1980, vol. 5, p. 1597.
Mandal, S.K. and Nag, K., J. Chem. Soc., Dalton Trans., 1983, vol. 11, p. 2429.
Dharmaraj, N., Viswanathamurthi, P., and Natarajan, K., Transition Met. Chem., 2001, vol. 26, p. 105.
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
Etaiw, S.E.H., El-Bendary, M.M. & Abdelazim, H. Synthesis, characterization, and biological activity of Cd(II) and Mn(II) coordination polymers based on pyridine-2,6-dicarboxylic acid. Russ J Coord Chem 43, 320–330 (2017). https://doi.org/10.1134/S1070328417050013
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070328417050013