Abstract
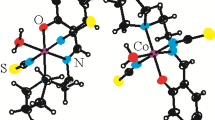
A new cobalt(II,III) complex, [CoIIIL2]2[Co II2 (HL)2(OH2)2(CH3OH)2] • 2H2O (I) and a new iron(III) complex, [FeIII(HL)2](NO3) (II), where L2– and HL– are the dianionic and monoanionic form of N'-(2-hydroxybenzylidene)-3-methylbenzohydrazide, respectively, have been prepared and characterized by elemental analyses, infrared and UV-Vis spectroscopy and single-cyrstal X-ray diffraction (CIF files CCDC nos. 1417971 (I), 1417979 (II)). Complex I crystallizes in the monoclinic space group P21/n with unit cell dimensions a = 16.1665(9), b = 14.5692(8), c = 19.086(1) Å, β = 96.347(1)°, V = 4467.9(4) Å3, Z = 2, R 1 = 0.0521, and wR 2 = 0.1411. Complex II crystallizes in the orthorhombic space group Pbcn with unit cell dimensions a = 12.475(1), b = 12.202(1), c = 18.859(2) Å, V = 2870.8(4) Å3, Z = 4, R 1 = 0.0796, and wR 2 = 0.1981. The metal atoms in the complexes are in octahedral coordination. Crystals of the complexes are stabilized by hydrogen bonds. The efficiency of the aroylhydrazone and the two complexes was evaluated against B. subtilis, S. aureus, E. coli, P. fluorescence, C. albicans and A. niger, with the complexes demonstrating enhanced activity relatively to the free ligand.
Similar content being viewed by others
References
Gavey, E.L. and Pilkington, M., Coord. Chem. Rev., 2015, vol. 296, p. 125.
Consiglio, G., Oliveri, I.P., Punzo, F., et al., Dalton Trans., 2015, vol. 44, no. 29, p. 13040.
Lachachi, M.B., Benabdallah, T., Aguiar, P.M., et al., Dalton Trans., 2015, vol. 44, no. 26, p. 11919.
Aono, Y., Yoshida, H., Katoh, K., et al., Inorg. Chem., 2015, vol. 54, no. 14, p. 7096.
Khorshidifard, M., Rudbari, H.A., Askari, B., et al., Polyhedron, 2015, vol. 95, p. 1.
Toledano-Magana, Y., Garcia-Ramos, J.C., Navarro-Olivarria, M., et al., Molecules, 2015, vol. 20, no. 6, p. 9929.
Kaplanek, R., Havlik, M., Dolensky, B., et al., Bioorg. Med. Chem., 2015, vol. 23, no. 7, p. 1651.
Rajitha, G., Prasad, K.V.S.R.G., Umamaheswari, A., et al., Med. Chem. Res., 2014, vol. 23, no. 12, p. 5204.
Altintop, M.D., Ozdemir, A., Ilgin, S., et al., Lett. Drug Des. Discov., 2014, vol. 11, no. 7, p. 833.
Altintop, M.D., Ozdemir, A., Turan-Zitouni, G., et al., Eur. J. Med. Chem., 2012, vol. 58, p. 299.
Kaplancikli, Z.A., Altintop, M.D., Ozdemir, A., et al., Lett. Drug. Des. Discov., 2012, vol. 9, no. 3, p. 310.
Blanot, D., Lee, J., and Girardin, S.E., Chem. Biol. Drug Des., 2012, vol. 79, no. 1, p. 2.
Chew, S.T., Lo, K.M., Sinniah, S.K., et al., RSC Advances, 2014, vol. 4, no. 106, p. 61232.
Sheng, G.-H., Han, X., You, Z.L., et al., J. Coord. Chem., 2014, vol. 67, no. 10, p. 1760.
Tsay, O.G., Manjare, S.T., Kim, H., et al., Inorg. Chem., 2013, vol. 52, no. 17, p. 10052.
Alagesan, M., Bhuvanesh, N.S.P., and Dharmaraj, N., Dalton Trans., 2013, vol. 42, no. 19, p. 7210.
Datta, A., Liu, P.-H., Huang, J.-H., et al., Polyhedron, 2012, vol. 44, no. 1, p. 77.
Al-Shaalan, N.H., Molecules, 2011, vol. 16, no. 10, p. 8629.
Hegazy, W.H. and Al-Motawaa, I.H., Synth. React. Inorg. Met.-Org. Nano-Met. Chem., 2011, vol. 41, no. 9, p. 1172.
El-Dissouky, A., Al-Fulaij, O., Awad, M.K., et al., J. Coord. Chem., 2010, vol. 63, no. 2, p. 330.
Zhang, M., Xian, D.-M., Li, H.-H., et al., Aust. J. Chem., 2012, vol. 65, no. 4, p. 343.
Ye, Y.-T., Niu, F., Sun, Y., et al., Chin. J. Inorg. Chem., 2015, vol. 31, no. 5, p. 1019.
Qu, D., Niu, F., Zhao, X.L., et al., Bioorg. Med. Chem., 2015, vol. 23, no. 9, p. 1944.
Huo, Y., Ye, Y.-T., Cheng, X.-S., et al., Inorg. Chem. Commun., 2014, vol. 45, p. 131.
Zhao, Y., Han, X., Zhou, X.-X., et al., Chin. J. Inorg. Chem., 2013, vol. 29, no. 4, p. 867.
Liu, Z.-X., Acta Crystallogr., Sect. E: Struct. Rep. Online, 2011, vol. 67, no. 12, p. o3439.
SMART and SAINT, Madison: Bruker AXS, Inc., 2002.
Sheldrick, G.M., SADABS, Program for Empirical Absorption Correction of Area Detector, Göttingen: Univ. of Göttingen, 1996.
Sheldrick, G.M., SHELXTL, Version 5.1, Software Reference Manual, Madison: Bruker AXS, Inc., 1997.
Meletiadis, J., Meis, J.F., Mouton, J.W., et al., J. Clin. Microbiol., 2000, vol. 38, no. 8, p. 2949.
Sebastian, M., Arun, V., Robinson, P.P., et al., J. Coord. Chem., 2010, vol. 63, no. 2, p. 307.
Shaabani, B., Khandar, A.A., Mobaiyen, H., et al., Polyhedron, 2014, vol. 80, p. 166.
Sreekanth, A., Kala, U.L., Nayar, C.R., et al., Polyhedron, 2004, vol. 23, no. 1, p. 41.
Bogdanovic, G.A., Leovac, V.M., Vojinovic-Jesic, L.S., et al., J. Serb. Chem. Soc., 2007, vol. 72, no. 1, p. 63.
Wu, L.-M., Teng, H.-B., Feng, X.-C., et al., Cryst. Growth Des., 2007, vol. 7, no. 7, p. 1337.
Sadhukhan, D., Ray, A., Pilet, G., et al., Bull. Chem. Soc. Jpn., 2011, vol. 84, no. 7, p. 764.
Charkoudian, L.K., Pham, D.M., Kwon, A.M., et al., Dalton Trans., 2007, no. 43, p. 5031.
Matoga, D., Szklarzewicz, J., Stadnicka, K., et al., Inorg. Chem., 2007, vol. 46, no. 22, p. 9042.
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
Yang, T., Niu, F., Li, L.X. et al. Synthesis, characterization, crystal structures, and antimicrobial activity of cobalt(II) and iron(III) complexes derived from N'-(2-hydroxybenzylidene)-3-methylbenzohydrazide. Russ J Coord Chem 42, 402–409 (2016). https://doi.org/10.1134/S1070328416050109
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070328416050109