Abstract
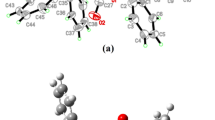
A new polymeric gold(I) diisobutyl dithiophosphate (Dtph), [Au2{S2P(O-iso-C4H9)2}2] n (I), was preparatively obtained and characterized by 13C and 31P MAS NMR spectroscopy and X-ray diffraction (CIF file CCDC no. 977818). Diagrams of the χ2 statistic were constructed from the complete 31P MAS NMR spectra and used to calculate the 31P chemical shift anisotropy (δ aniso = δ zz − δ iso ) and the asymmetry parameter η = (δ yy − δ xx )/(δ zz − δ iso ). The main structural unit of complex I is the noncentrosymmetric dinuclear molecule [Au2{S2P(O-iso-C4H9)2}2], in which the gold atoms are linked by two bridging ligands Dtph. The central cyclic structural fragment of the dimer [Au2S4P2] is additionally stabilized by the intramolecular aurophilic interaction Au⋯Au. Further supramolecular self-organization of the complex involves intermolecular aurophilic bonds Au⋯Au that serve to unite adjacent dinuclear molecules [Au2{S2P(O-iso-C4H9)2}2] with different spatial orientations into the polymer chains ([Au2{S2P(O-iso-C4H9)2}2]) n . The thermal behavior of complex I was examined by synchronous thermal analysis under argon. The character of the thermolysis of the complex to reduced metallic gold as a final product was determined.
Similar content being viewed by others
References
Lawton, S.L., Rohrbaugh, W.J., and Kokotailo, G.T., Inorg. Chem., 1972, vol. 11, no. 9, p. 2227.
Lee, Y.-A., McGarrah, J.E., Lachicotte, R.J., and Eisenberg, R., J. Am. Chem. Soc., 2002, vol. 124, no. 36, p. 10662.
Ivanov, A.V., Lutsenko, I.A., Korneeva, E.V., et al., Rus. J. Coord. Chem., 2012, vol. 38, no. 6, p. 430.
Ivanov, A.V., Korneeva, E.V., Lutsenko, I.A., et al., J. Mol. Struct., 2013, vol. 1034, p. 152.
Schmidbaur, H., Gold Bull., 2000, vol. 33, no. 1, p. 3.
Uson, R., Laguna, A., Laguna, M., et al., Chem. Commun., 1988, no. 11, p. 740.
Pathaneni, S.S. and Desiraju, G.R., Dalton Trans., 1993, no. 2, p. 319.
Cao, L., Jennings, M.C., and Puddephatt, R.J., Inorg. Chem., 2007, vol. 46, no. 4, p. 1361.
Staples, R.J. and Fackler, Jr., J.P., Inorg. Chem. Commun., 1998, vol. 1, no. 2, p. 51.
Zyl van, W.E., Lopez-de-Luzuriaga, J.M., and Fackler, J.P., Jr., J. Mol. Struct., 2000, vol. 516, no. 1, p. 99.
Zyl van, W.E., Lopez-de-Luzuriaga, J.M., and Mohamed, A.A., et al., Inorg. Chem., 2002, vol. 41, no. 17, p. 4579.
Maspero, A., Kani, I., Mohamed, A.A., et al., Inorg. Chem., 2003, vol. 42, no. 17, p. 5311.
Karakus, M., Lonnecke, P., Hildebrand, M., and Hey-Hawkins, E., Z. Anorg. Allg. Chem., 2011, vol. 637, nos. 7–8, p. 983.
Siasios, G. and Tiekink, E.R.T., Z. Kristallogr., 1995, vol. 210, p. 698.
Preisenberger, M., Bauer, A., Schier, A., and Schmidbaur, H., Dalton Trans., 1997, no. 24, p. 4753.
Gysling, H.J., Blanton, T.N., McMillan, S.M., and Lushington, K.J., Z. Kristallogr., 1999, vol. 214, p. 309.
Zyl van, W.E., Lopez-de-Luzuriaga, J.M., and Fackler, J.P., Jr., and Staples, R.J., Can. J. Chem., 2001, vol. 79, nos. 5–6, p. 896.
Miller, J.B. and Burmeister, J.L., Synth. React. Inorg. Met.-Org. Chem., 1985, vol. 15, no. 2, p. 223.
Pines, A., Gibby, M.G., and Waugh, J.S., J. Chem. Phys., 1972, vol. 56, no. 4, p. 1776.
Press, W.H., Teukolsky, S.A., Vetterling, W.T., and Flannery, B.P., Numerical Recipes, Cambridge: Univ. Press, 1994.
Hodgkinson, P. and Emsley, L., J. Chem. Phys., 1997, vol. 107, no. 13, p. 4808.
Antzutkin, O.N., Lee, Y.K., and Levitt, M.H., J. Magn. Reson., 1998, vol. 135, no. 1, p. 144.
Wolfram, S., The Mathematica Book, Cambridge: Wolfram Media/Cambridge Univ., 1999, p. 1470.
APEX2, Madison (WI, USA): Bruker AXS, 2010.
SAINT, Madison (WI, USA): Bruker AXS, 2010.
Sheldrick, G.M., Acta Crystallogr., Sect. A: Found. Crystallogr., 2008, vol. 64, no. 1, p. 112.
Larsson, A.-C., Ivanov, A.V., Forsling, W., et al., J. Am. Chem. Soc., 2005, vol. 127, no. 7, p. 2218.
Ivanov, A.V., Antzutkin, O.N., Larsson, A.-C., et al., Inorg. Chim. Acta, 2001, vol. 315, no. 1, p. 26.
Ivanov, A.V., Loseva, O.V., Ivanov, M.A., et al., Russ. J. Inorg. Chem., 2007, vol. 52, no. 10, p. 1595.
Ivanov, A.V., Gerasimenko, A.V., Antzutkin, O.N., and Forsling, W., Inorg. Chim. Acta, 2005, vol. 358, no. 9, p. 2585.
Larsson, A.-C., Ivanov, A.V., Antzutkin, O.N., et al., Inorg. Chim. Acta, 2004, vol. 357, no. 9, p. 2510.
Larsson, A.-C., Ivanov, A.V., Pike, K.J., et al., J. Magn. Reson., 2005, vol. 177, no. 1, p. 56.
Ivanov, A.V., Zinkin, S.A., Gerasimenko, A.V., et al., Rus. J. Coord. Chem., 2007, vol. 33, no. 1, p. 20.
Rodina, T.A., Ivanov, A.V., Konfederatov, V.A., et al., Russ. J. Inorg. Chem., 2009, vol. 54, no. 11, p. 1779.
Ivanov, A.V., Konfederatov, V.A., Gerasimenko, A.V., Larsson, A.C., Rus. J. Coord. Chem., 2009, vol. 35, no. 11, p. 857.
Pauling, L., The Nature of the Chemical Bond and the Structure of Molecules and Crystals, London: Cornell Univ., 1960.
Bondi, A., J. Phys. Chem., 1964, vol. 68, no. 2, p. 441.
Bondi, A., J. Phys. Chem., 1966, vol. 70, no. 9, p. 3006.
Evans, D.G. and Boeyens, J.C.A., Acta Crystallogr., Sect. B: Struct. Sci., 1988, vol. 44, no. 6, p. 663.
Lawton, S.L. and Kokotailo, G.T., Inorg. Chem., 1969, vol. 8, no. 11, p. 2410.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © E.V. Korneeva, T.A. Rodina, A.V. Ivanov, A.V. Gerasimenko, A.-C. Larsson, 2014, published in Koordinatsionnaya Khimiya, 2014, Vol. 40, No. 10, pp. 631–640.
Rights and permissions
About this article
Cite this article
Korneeva, E.V., Rodina, T.A., Ivanov, A.V. et al. Polymeric gold(I) diisobutyl dithiophosphate, [Au2{S2P(O-iso-C4H9)2}2]n: Synthesis, supramolecular self-organization (a role of aurophilic interaction), 13C and 31P MAS NMR spectroscopy, and thermal behavior. Russ J Coord Chem 40, 748–756 (2014). https://doi.org/10.1134/S1070328414100042
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070328414100042