Abstract
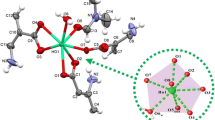
Compounds p-HOOCC6F4COOH · H2O (H2L · H2O), [Tb2(H2O)4(L)3 · 2H2O] n (I), and Tb2(Phen)2(L)3 · 2H2O (II) are synthesized. According to the X-ray structure analysis data, the crystal structure of H2L · H2O is built of centrosymmetric molecules H2L and molecules of water of crystallization. The crystal structure of compound I is built of layers of coordination 2D polymer [Tb2(H2O)4(L)3] n and molecules of water of crystallization. The ligands are the L2− anions performing both the tetradentate bridging and pentadentate bridging-chelating functions. The coordination polyhedron TbO9 is a distorted three-capped trigonal prism. Acid H2L manifests photoluminescence in the UV region (λmax = 368 nm). Compounds I and II have the green luminescence characteristic of the Tb3+ ions, and the band with λmax = 545 nm (transition 5 D 4→ 7 F 5) is maximum in intensity. The photoluminescence intensity of compound II is higher than that for compound I.
Similar content being viewed by others
References
De Sa, G.F., Malta, O.L., de Mello, Donega, C., et al., Coord. Chem. Rev., 2000, vol. 196, p. 165.
Katkova, M.A., Vitukhnovskii, A.G., and Bochkarev, M.N., Usp. Khim., 2005, vol. 24, no. 12, p. 1193.
Bünzli, J.-C.G., Acc. Chem. Res., 2006, vol. 39, p. 53.
Armelao, L., Quici, S., Barigelletti, F., et al., Coord. Chem. Rev., 2010, vol. 254, p. 487.
Katkova, M.A. and Bochkarev, M.N., Dalton Trans., 2010, vol. 39, p. 6599.
Katkova, M.A., Borisov, A.V., Fukin, G.K., et al., Inorg. Chim. Acta, 2006, vol. 359, no. 13, p. 4289.
Faustino, W.M., Malta, O.L., Teotonio, E.E.S., et al., J. Phys. Chem. A, 2006, vol. 110, p. 2510.
Regulacio, M.D., Pablico, M.H., Vasquez, J.A., et al., Inorg. Chem., 2008, vol. 47, no. 5, p. 1512.
Larionov, S.V., Varand, V.L., Klevtsova, R.F., et al., Russ. J. Coord. Chem., 2008, vol. 34, no. 12, p. 931.
Varand, V.L., Uskov, E.M., Korol’kov, I.V., and Larionov, S.V., Russ. J. Gen. Chem., 2009, vol. 79, no. 2, p. 228.
Kokina, T.E., Klevtsova, R.F., Uskov, E.M., et al., Zh. Strukt. Khim., 2010, vol. 51, no. 5, p. 976.
Peshkova, S.B., Topilova, Z.M., Lozinskii, O.M., et al., Zh. Anal. Khim., 1997, vol. 52, no. 9, p. 939.
Panin, E.S., Kavun, V.Ya., Sergienko, V.I., and Bukvetskii, B.V., Koord. Khim., 1985, vol. 11, no. 11, p. 1539.
Larionov, S.V., Kirichenko, V.N., Rastorguev. A.A., et al., Russ. J. Coord. Chem., 1997, vol. 23, no. 6, p. 432.
Smagin, V.P., Kotvanova, M.K., and Ulanskaya, O.A., Russ. J. Coord. Chem., 1998, vol. 24, no. 11, p. 818.
Kalinovskaya, N.V., Karasev, V.E., and Pyatkina, A.N., Russ. J. Inorg. Chem., 1999, vol. 44, no. 3, p. 380.
Rastorguev, A.A., Remova, A.A., Romanenko, G.V., et al., Zh. Strukt. Khim., 2001, vol. 42, p. 907.
Baria, B., Baggio, R., Garland, M.T., et al., Inorg. Chim. Acta, 2003, vol. 346, p. 187.
Larionov, S.V., Glinskaya, L.A., Leonova, T.G., et al., Russ. J. Coord. Chem., 2009, vol. 35, no. 11, p. 798.
Glinskaya, L.A., Leonova, T.G., Klevtsova, R.F., and Larionov, S.V., Zh. Strukt. Khim., 2010, vol. 51, no. 3, p. 610.
Chen, B., Zapata, F., Qian, G., et al., Inorg. Chem., 2006, vol. 45, no. 22, p. 8883.
Mac Neill C.M., Day, C.S. Marts, A., et al., Inorg. Chim. Acta, 2011, vol. 365, p. 196.
Sintezy ftoroorganicheskikh soedinenii (Syntheses of Organofluorine Compounds), Knunyants, I.L. and Yakobson, G.G., Eds., Moscow: Khimiya, 1973, p. 173.
Sheldrik, G.M., Acta Crystallogr., Sect. A: Found. Crystallogr., 2008, vol. 64, p. 112.
Schajor, W., Post, H., Grosescu, R., and Haeberlen, U., J. Magn. Res., 1983, vol. 53, p. 213.
Kraus, W. and Nolze, G., J. Appl. Crystallogr., 1996, vol. 29, p. 301.
Poluektov, N.S., Kononenko, L.I., Efryushina, N.P., and Bel’tyukova, S.V., Spektrofoto-metricheskie i lyuminestsentnye metody opredeleniya lantanoidov (Spectrophotometric and Luminescence Methods of Lanthanide Determination), Kiev: Nauk. dumka, 1989.
Accorsi, G., Listorti, A., Yoosaf, K., and Armaroli, N., Chem. Soc. Rev., 2009, vol. 38, p. 1690.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © S.V. Larionov, L.I. Myachina, L.A. Glinskaya, I.V. Korol’kov, E.M. Uskov, O.V. Antonova, V.M. Karpov, V.E. Platonov, V.P. Fadeeva, 2012, published in Koordinatsionnaya Khimiya, 2012, Vol. 38, No. 12, pp. 827–833.
Rights and permissions
About this article
Cite this article
Larionov, S.V., Myachina, L.I., Glinskaya, L.A. et al. Syntheses and structures of p-HOOCC6F4COOH · H2O (H2L · H2O) and luminescent coordination polymers [Tb2(H2O)4(L)3 · 2H2O] n and Tb2(Phen)2(L)3 · 2H2O. Russ J Coord Chem 38, 717–723 (2012). https://doi.org/10.1134/S1070328412110036
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070328412110036