Abstract
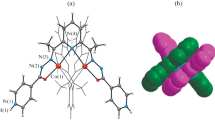
A new Schiff base copper(II) complex, {[CuL(H2O)2][CuL(H2O)]3 · 4H2O · C3H7NO} n (H2L = (Z)-2-(2-hydroxy-3-methoxybenzylideneamino)-4-(methylthio)butanoic acid), was synthesized and characterized by IR, UV, and X-ray diffraction single-crystal analysis. The crystal belongs to the triclinic crystal system, space group with cell parameters a = 5.2027(5) Å, b = 16.6916(16) Å, c = 20.237(2) Å, α = 88.895(10)°, β = 84.127(1)°, γ = 83.577(10)°, V = 1737.2(3) Å3, Z = 1, F(000) = 848, S = 1.042, ρcalcd = 1.561 g cm−3, μ = 1.411 mm−1, the final R 1 = 0.0760 and wR 2 = 0.2318 for 6030 observed reflections (I > 2σ(I)). The crystal structure of the complex contains two independent units with different coordination environments. In independent unit 1, the Cu(1) is five-coordinated by one nitrogen atom and two oxygen atoms from the Schiff base ligand and two oxygen atoms from two water molecules to form a distorted square pyramid geometry. On the other hand, in independent unit 2, the Cu(2) is five-coordinated and possesses a slightly distorted square-pyramidal coordination geometry, defined by one nitrogen atom, one hydroxyl oxygen atom, two carboxylate oxygen atoms in two different ligands, and one oxygen atom of the water molecule. The complex forms a one-dimensional chain polymer in the xy plane through carboxyl oxygen atoms in the Schiff base ligands.
Similar content being viewed by others
References
Shi, Q., Xu, L., Ji, J., et al., Inorg. Chem. Commun., 2004, vol. 7, p. 1254.
You, Z.L., Zhu, H.L., Liu, W.S., et al., Z. Anorg. Allg. Chem., 2004, vol. 630, p. 1617.
Golcu, A., Tumer, M., Demirelli, H., et al., Inorg. Chim. Acta, 2005, vol. 358, p. 1785.
Ziessel, R., Coord. Chem. Rev., 2001, vol. 195, p. 216.
Choudhury, C.R., Dey, S.K., Mondal, N., et al., J. Chem. Crystallogr., 2002, vol. 31, p. 57.
West, D.X., Gebremedhin, H., Butcher, R.J., et al., Polyhedron, 1993, vol. 12, p. 2489.
Chandra, S. and Sangeetika, X., Spectrochim. Acta, A, 2004, vol. 60, p. 147.
Kimura, E., Wada, S., Shiyonoya, M., et al., Inorg. Chem., 1994, vol. 96, p. 770.
Lambert, S.L., Spiro, C., Gagne, R.R., et al., Inorg. Chem., 1982, vol. 21, p. 68.
Teicher, B.A., Abrams, M.J., and Rosbe, K.W., Cancer Res., 1990, vol. 50, p. 6971.
Srinivasn, K., Shankaranarayanan, K., Thangavelu, S., et al., Cryst. Growth, 2000, vol. 212, p. 246.
Rao, R.V., Rao, C.P., and Wegelius, E.K., J. Chem. Crystallogr., 2003, vol. 33, p. 139.
Qiao, Y.H., Yue, M., Sun, M., et al., Chin. J. Appl. Chem., 2003, vol. 20, p.192.
Ye, G.R., Chai, Y.Q., Ruo, R.Y., et al., Anal. Sci., 2007, vol. 23, p. 171.
Fan, Y.H., Bi, S.Y. Li, Y.Y., et al., Rus. J. Coord. Chem., 2008, vol. 33, p. 772.
Lumme, P. and Elo, H., Inorg. Chim. Acta, 1984, vol. 94, p. 241.
Antony, F.M., Mahony, R.S., and Joyce, M.W., Croat. Chem. Acta, 1999, vol. 72, p. 685.
Xiao, Y., Bi, C.F., Fan, Y.H., et al., Int. J. Oncol., 2008, vol. 33, p. 1073.
Sheldrick, G.M., SHELXTL-97, Program for Crystal Structure Refinement, Göttingen (Germany): Univ. of Göttingen, 1997.
Addison, A.W., Rao, T.N., Reedijk, J., et al., Dalton Trans., 1984, p. 1349.
Lewis, D.L., McGregor, K.T., Hatfield, W.E., et al., Inorg. Chem., 1974, vol. 13, p. 1013.
Mandal, S.K., Thompson, L.K., Newlands M.J., et al., Inorg. Chem., 1990, vol. 29, p. 1324.
Sangeetha, N.R., Baradi, K., Gupta, R., et al., Polyhedron, 1999, vol. 18, p. 1425.
Bi, C.F. and Fan, Y.H., Synth. React. Inorg. Met.-Org. Chem., 2004, vol. 34, p. 687.
Kasumov, V.T., Ibrahim, U., and Ahmed, B., J. Fluorine Chem., 2010, vol. 131, p. 59.
Bernadette, S.C., Michael, D., Agnieszka, F., et al., Polyhedron, 2010, vol. 29, p. 813.
Manimaran, A., Prabhakaran, R., Deepa, T., et al., Appl. Organomet. Chem., 2008, vol. 22, p. 353.
Arslan, F., Odabasoglu, M., Oelmez, H., and Büyükgüngör, O., Polyhedron, 2009, vol. 28, p. 2943.
Wang, Y.F., Liu, J.F., Xian, H.D., et al., Molecules, 2009, vol. 14, p. 2582.
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
Wang, Q., Bi, C.F., Fan, Y.H. et al. A novel copper(II) complex with schiff base derived from o-vanillin and L-methionine: Syntheses and crystal structures. Russ J Coord Chem 37, 228–234 (2011). https://doi.org/10.1134/S1070328411020138
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070328411020138