Abstract
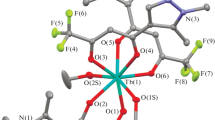
A terbium p-methyl benzoate complex with 1,10-phenanthroline, [Tb(p-MBA)3(Phen)]2 (p-MBA = p-methyl benzoate and Phen = 1,10-phenanthroline), has been prepared and structurally characterized by X-ray diffraction. It crystallizes in triclinic system, space group P \( \bar 1 \) with a = 12.8064(12), b = 13.3589(12), c = 19.8277(19) Å, α = 91.668(2)°, β = 97.775(2)°, γ = 106.312(2)°, C72H58N4O12Tb2, M r = 1489.06, V = 3217.6(5) Å3, Z = 2, ρ c = 1.537 g/cm3, μ(MoK α) = 2.246 mm−1, F(000) = 1488, the final R = 0.0622 and wR = 0.0962 for 14128 independent reflections with R int = 0.0843. It consists of two types of crystallographically independent dimeric molecules [Tb(p-MBA)3(Phen)]2 noted as [Tb-1] and [Tb-2]. In the dimeric molecule [Tb-1], each Tb(III) is eight-coordinated with one Phen molecule, two bridging carboxylate groups, and two bidentate chelating carboxylate groups, while in [Tb-2] each Tb(III) is eight-coordinated with one Phen molecule, four bridging carboxylate groups, and one bidentate chelating carboxylate group. The title complex shows intense green luminescence under UV light at room temperature.
Similar content being viewed by others
References
Galaup, C., Picard, C., Cathala, B., et al., Chim. Acta, 1999, vol. 82, p. 543.
Sabbatini, N., Guardigli, M., and Lehn, J.M., Coord. Chem. Rev., 1993, vol. 123, p. 201.
Nassar, E.J., Calefi, P.S., Rosa, I.L.V., et al., J. Alloys Compd., 1998, vols. 275–277, p. 838.
Bunzli, J.C.G. and Ihringer, F., Inorg. Chim. Acta, 1996, vol. 246, p. 195.
Sharma, P.K., Doorn, A.R., and Staring, A.G.J., J. Lumin., 1994, vol. 62, p. 219.
Lamture, J.B., Zhou, Z.H., Kumar, A.S., et al., Inorg. Chem., 1995, vol. 34, p. 864.
Wang, Y.B., Zheng, X.J., Zhuang, W.J., et al., Eur. J. Inorg. Chem., 2003, p. 3572.
Lam, A.W.H., Wong, W.T., Gao, S., et al., Eur. J. Inorg. Chem., 2003, p. 149.
Wang, Y.B., Zheng, X.J., Zhuang, W.J., et al., Eur. J. Inorg. Chem., 2003, p. 1355.
Li, X., Zhang, Z.Y., and Zou, Y.Q., Eur. J. Inorg. Chem., 2005, p. 2909.
Yuan, L.J., Yin, M.C., Yuan, E.T., et al., Inorg. Chim. Acta, 2004, no. 357, p. 89.
Hemmila, I., J. Alloys Compd., 1995, no. 225, p. 480.
Coates, J., Sammes, P.G., Yahioglu, G., et al., Chem. Commun., 1994, p. 2311.
Kido, J., Ikeda, W., Kimura, M., et al., J. Appl. Phys., 1996, vol. 35, p. 394.
Sun, M., Xin, H., Wang, K.Z., et al., Chem. Commun., 2003, p. 702.
Xin, H., Li, F.Y., Shi, M., et al., J. Am. Chem. Soc., 2003, vol. 125, p. 7166.
Andre, N., Jensen, T.B., Scopelliti, R., et al., Inorg. Chem., 2004, vol. 43, p. 515.
Quici, S., Cavazzini, M., Marzanni, G., et al., Inorg. Chem., 2005, vol. 44, p. 529.
Zhang, Z.H., Okamura, T.A., Hasegawa, Y., et al., Inorg. Chem., 2005, vol. 44, p. 6219.
Liu, W.S., Jiao, T.Q., Li, Y.Z., et al., J. Am. Chem. Soc., 2004, vol. 126, p. 2280.
Guo, X.D., Zhu, G.S., Fang, Q.R., et al., Inorg. Chem., 2005, vol. 44, p. 3850.
Bettencourt-Dias, A.D., Inorg. Chem., 2005, vol. 44, p. 2734.
Soares-Santos, P.C.R. and Nogueira, H.I.S., Almeida Paz, F.A., et al., Eur. J. Inorg. Chem., 2003, p. 3609.
Wang, R.F., Wang, S.P., and Zhang, J.J., J. Mol. Struct., 2003, no. 648, p. 151.
Wang, R.F., Wang, S.P., Shi, S.K., et al., Chin. J. Struct. Chem., 2004, vol. 23, no. 11, p. 1300.
Sheldrick, G.M., SHELXS-97, Program for the Solution of Crystal Structures, Göttingen (Germany): Univ. of Göttingen, 1997.
Sheldrick, G.M., SHELXL-97, Program for the Refinement of Crystal Structures, Göttingen (Germany): Univ. of Göttingen, 1997.
Author information
Authors and Affiliations
Corresponding author
Additional information
The article is published in the original.
Rights and permissions
About this article
Cite this article
Wang, S.P., Xu, L.J., Gao, Z.H. et al. Crystal structure and luminescence of a terbium complex with p-methyl benzoate and 1,10-phenanthroline. Russ J Coord Chem 36, 786–792 (2010). https://doi.org/10.1134/S1070328410100106
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070328410100106