Abstract
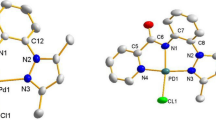
The alkylation of ethylenediamine with allyl bromide in the presence of a fourfold (with respect to ethylenediamine) molar amount of NaHCO3 in acetone with an ethanol admixture (15: 1) affords LBr2 · 2H2O (I), where L2+ is the N,N,N,N′,N′,N′-hexaallylethylenediaminium cation. Single crystals of complexes L[CuII(Br0.45Cl3.55)] (II), L[Cu I4 (Br4.55Cl1.45)] (III), and L[Cu I4 Br6] (IV) are prepared by ac electrochemical synthesis from an ethanolic solution of LBr2 · 2H2O, CuCl2 · 2H2O (or CuBr2) at copper wire electrodes. The crystal structures of compounds I–IV are determined by X-ray diffraction analysis. The crystals of complex I are monoclinic: space group P21/n, a = 8.544(3), b = 10.404(3), c = 13.350(4) Å, β = 97.29(3)°, V = 1177.2(6) Å3, Z = 2. The bromine anions in compound I are bonded to the L2+ cations and water molecules through hydrogen contacts (E)H…Br (E = O, C) of 2.57(3)–2.86(3) Å. The crystals of compounds II–IV are triclinic: space group P \( \bar 1 \). For II: a = 8.762(4), b = 9.163(4), c = 16.500(6) Å, α = 95.62(4)°, β = 96.39(4)°, γ = 111.46(4)°, V = 1211.4(9) Å3, Z = 2; for III: a = 9.074(4), b = 9.435(4), c = 9.829(5) Å, α = 116.12(4)°, β = 104.14(4)°, γ = 100.22(4)°, V = 692.3(6) Å3, Z = 1; for IV isostructural III: a = 9.084(4), b = 9.404(4), c = 9.869(4) Å, α = 116.31(3)°, β = 104.00(3)°, γ = 100.37(3)°, V = 692.1(5) Å3, Z = 1. Unlike the isolated tetrahedral CuX 2−4 anion in structure II, an original chain anion (Cu4X 2−6 ) n is observed in the structures of π complexes III and IV.
Similar content being viewed by others
References
Hong-Jan Mao, Xiao-Qing Shen, Gang Li, et al., Polyhedron, 2004, vol. 23, no. 11, p. 1961.
Anacona, J.R., Gutierrez, T., and Rodriguez-Barbarin, C., Monatsh. Chem., 2004, vol. 135, no. 7, p. 785.
Ball, R.D., Hall, D., Rickard, C.E.F., and Waters, T.N., J. Chem. Soc., Sect. A, 1967, p. 1435.
Pasquali, M., Floriani, C., and Gaetani-Manfredotti, A., Inorg. Chem., 1980, vol. 19, no. 5, p. 1191.
Engelhardt, L.M., Papasergio, R.I., and White, A.H., Aust. J. Chem., 1984, vol. 37, no. 11, p. 2207.
Shkurenko, A.A., Davydov, V.N., and Mys’kiv, M.G., Koord. Khim., 2003, vol. 29, no. 6, p. 475 [Russ. J. Coord. Chem. (Engl. Transl.), vol. 29, no. 6, p. 445].
Pavlyuk, A.V., Mykhalichko, B.M., and Mys’kiv, M.G., Koord. Khim., 2004, vol. 30, no. 3, p. 172 [Russ. J. Coord. Chem. (Engl. Transl.), vol. 30, no. 3, p. 159].
Mel’nyk, O.P., Schollmeyer, D., Olijnyk, V.V., and Filinchuk, Ya.E., Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 2001, vol. 57, no. 2, p. 151.
CrysAlis RED, Oxford: Diffraction Ltd. Version 1.171.31.8 (Release 12-01-2007. CrysAlis 171.NET).
Clark, R.C. and Reid, J.S., Acta Crystallogr., Sect. A: Found. Crystallogr., 1995, vol. 51, no. 6, p. 887.
Sheldrick, G., SHELXL-97. Program for Refinement of Crystal Structures. Göttingen (Germany): Univ. of Göttingen, 1997.
Pavlyuk, A.V., Davydov, V.N., and Mys’kiv, M.G., Koord. Khim., 2003, vol. 29, no. 3, p. 213 [Russ. J. Coord. Chem. (Engl. Transl.), vol. 29, no. 3, p. 199].
Mys’kiv, M.G., Oliinik, V.V., Zavalii, P.Yu., et al., Metalloorg. Khim., 1989, vol. 2, no. 6, p. 1225.
Oliinik, V.V., Mys’kiv, M.G., Mazus, M.D., et al., Zh. Strukt. Khim., 1992, vol. 33, no. 1, p. 121.
Goreshnik, E.A. and Mys’kiv, M.G., Koord. Khim., 2003, vol. 29, no. 12, p. 936 [Russ. J. Coord. Chem. (Engl. Transl.), vol. 29, no. 2, p. 871].
Goreshnik, E.A., Mys’kiv, M.G., Mazus, M.D., et al., Kristallografiya, 1992, vol. 37, no. 1, p. 100.
Mys’kiv, M.G., Goreshnik, E.A., Pecharskii, V.K., and Oliinik, V.V., Zh. Strukt. Khim., 1994, vol. 35, no. 1, p. 90.
Olijnyk, V., Glowiak, T., and Mys’kiv, M., J. Chem. Crystallogr., 1995, vol. 25, no. 10, p. 621.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © M.M. Monchak, A.V. Pavlyuk, V.V. Kinzhibalo, M.G. Mys’kiv, 2009, published in Koordinatsionnaya Khimiya, 2009, Vol. 35, No. 6, pp. 414–419.
Rights and permissions
About this article
Cite this article
Monchak, M.M., Pavlyuk, A.V., Kinzhibalo, V.V. et al. New π ligand N,N,N,N′,N′,N′-hexaallylethylenediaminium (L2+): Synthesis and crystal structures of LBr2 · 2H2O and its cuprocomplexes L[CuII(Br0.45Cl3.55)], L[Cu I4 (Br4.55Cl1.45)], and L[Cu I4 Br6]. Russ J Coord Chem 35, 405–410 (2009). https://doi.org/10.1134/S1070328409060037
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070328409060037