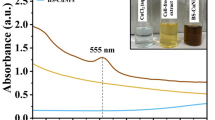
Abstract
Objective: With the molecular formula C8H6S, benzothiophene is an appealing synthon showing a wide range of biological activities such as antifungal, antibacterial, antitumor and anticancer properties. Due to increasing concern about the resistance gained by the microorganisms against existing antimicrobial agents, our group is interested in revealing the cellular target site of these compounds. Methods: As a part of our efforts on determination of effect of antibacterial agents, in this study, we focused on the benzothiophene Schiff bases. Antibacterial activity of bis(benzo[b]thiophene-2-yl)alkylmethanimine derivatives was studied via minimum inhibitory concentration measurements. Results and Discussion: One of the compounds, compound (I) (N,N′-(propane-1,3-diyl)bis(1-(benzo[b]thiophene-2-yl))methanimine), proved to be highly active against both Gram-positives; Staphylococcus aureus, Bacillus subtilis, Enterococcus faecalis, Bacillus cereus; and Gram-negatives Escherichia coli, Pseudomonas aeruginosa, Salmonella enteritidis, Shigella flexneri. In order to reveal the effect of this compound on bacterial cytoplasmic membrane, we measured the extracellular conductivity increase upon treatment. Compound (I), showing high antibacterial activity caused a sudden increase of extracellular conductivity due to ion leakage from bacterial cells. In contrast, inactive benzothiophene derivatives did not cause any conductivity increase. Conclusions: We propose that benzothiophene Schiff base (I) disrupts bacteria cytoplasmic membrane integrity, and this action contributes to its antibacterial activity.





Similar content being viewed by others
DATA AVAILABILITY
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
Bugg, T.D., Braddick, D., Dowson, C.G., and Roper, D.I., Trends Biotechnol., 2011, vol. 29, pp. 167–173. https://doi.org/10.1016/j.tibtech.2010.12.006
Pasquina, L.W., Santa Maria, J.P., and Walker, S., Curr. Opin. Microbiol., 2013, vol. 16, pp. 531–537. https://doi.org/10.1016/j.mib.2013.06.014
Hancock, R.E., Trends Microbiol., 1997, vol. 5, p. 37. https://doi.org/10.1016/S0966-842X(97)81773-8
Efremov, A.A., Zykova, I.D., Senashova, V.A., Grodnitckaya, I.D., and Pashenova, N.V., Russ. J. Bioorg. Chem.,2021, vol. 47, pp. 1439–1444.
Epand, R.M. and Epand, R.F., Biochim. Biophys. Acta (BBA)-Biomembranes, 2009, vol. 1788, pp. 289–294. https://doi.org/10.1016/j.bbamem.2008.08.023
Epand, R.M., Walker, C., Epand, R.F., and Magarvey, N.A., Biochim. Biophys. Acta (BBA)-Biomembranes, 2016, vol. 1858, pp. 980–987. https://doi.org/10.1016/j.bbamem.2015.10.018
Belete, T.M., Human Microbiome J., 2019, vol. 11, p. 100052. https://doi.org/10.1016/j.humic.2019.01.001
Şahal, H., İdil, Ö., Canpolat, E., and Özkan, M., Russ. J. Bioorg. Chem., 2023, vol. 49, pp. 602–609.
Alagarsamy, V., Narendhar, B., Chitra, K., Sriram, D., Sarvanan, G., and Solomon, V.R., Russ. J. Bioorg. Chem., 2022, vol. 48, pp. 1221–1229.
Amirkhanov, N.V., Bardasheva, A.V., Tikunova, N.V., and Pyshnyi, D.V., Russ. J. Bioorg. Chem., 2021, vol. 47, pp. 681–690.
Skrzyńska, A., Albrecht, A., and Albrecht, Ł., Adv. Synth. Cat., 2016, vol. 358, pp. 2838–2844. https://doi.org/10.1002/adsc.201600269
Jordan, V.C., J. Med. Chem., 2003, vol. 46, pp. 1081–1111. https://doi.org/10.1021/jm020450x
Martorana, A., Gentile, C., Perricone, U., Piccionello, A.P., Bartolotta, R., Terenzi, A., and Lauria, A., Eur. J. Med. Chem., 2015, vol. 90, pp. 537–546. https://doi.org/10.1016/j.ejmech.2014.12.002
Ardiansah, B., J. Appl. Pharm. Sci., 2019, vol. 9, pp. 117–129. https://doi.org/10.7324/JAPS.2019.90816
Naganagowda, G., Thanyongkit, P., and Petsom, A., J. Chil. Chem. Soc., 2012, vol. 57, pp. 1043–1047. https://doi.org/10.4067/S0717-97072012000100019
Meena, V.K., Patidar, A.K., and Bhatnagar, M., J. Adv. Sci. Res., 2020, vol. 11, pp. 228–231.
Jagtap, V.A. and Agasimundin, Y.S., J. Pharm. Res., 2015, vol. 9, pp. 10–14.
Romagnoli, R., Baraldi, P.G., Carrion, M.D., Cara, C.L., Preti, D., Fruttarolo, F., and Di Cristina, A., J. Med. Chem., 2007, vol. 50, pp. 2273–2277. https://doi.org/10.1021/jm070050f
Sweidan, K., Engelmann, J., Abu Rayyan, W., Sabbah, D., Abu Zarga, M., Al-Qirim, T., and Shattat, G., Lett. Drug Des. and Discov., 2015, vol. 12, pp. 417–429.
Fakhr, I.M., Radwan, M.A., El-Batran, S., ElSalam, O.M.A., and El-Shenawy, S.M., Eur. J. Med. Chem., 2009, vol. 44, pp. 1718–1725. https://doi.org/10.1016/j.ejmech.2008.02.034
Keri, R.S., Chand, K., Budagumpi, S., Balappa, S., Somappa, S., Patil, A., and Nagaraja, B.M., Eur. J. Med. Chem., 2017, vol. 138, pp. 1002–1033. https://doi.org/10.1016/j.ejmech.2017.07.038
Berrade, L., Aisa, B., Ramirez, M.J., Galiano, S., Guccione, S., Moltzau, L.R., and Aldana, I., J. Med. Chem., 2011, vol. 54, pp. 3086–3090. https://doi.org/10.1021/jm2000773
Mourey, R.J., Burnette, B.L., Brustkern, S.J., Daniels, J.S., Hirsch, J.L., Hood, W.F., and Schindler, J.F., J. Pharmacol. Exp. Ther., 2010, vol. 333, pp. 797–807. https://doi.org/10.1124/jpet.110.166173
Qin, Z., Kastrati, I., Chandrasena, R.E.P., Liu, H., Yao, P., Petukhov, P.A., and Thatcher, G.R., J. Med. Chem., 2007, vol. 50, pp. 2682–2692. https://doi.org/10.1021/jm070079j
Naganagowda, G. and Petsom, A., J. Sulfur Chem., 2011, vol. 32, pp. 223–233. https://doi.org/10.1080/17415993.2011.575943
Gujjarappa, R., Kabi, A.K., Vodnala, N., Tyagi, U., Kaldhi, D., and Malakar, C.C., Nanostruct. Biomat. Mat. Horizons: From Nat. Nanomat., Swain, B.P. (Ed) Springer, Singapore, 2022 https://doi.org/10.1007/978-981-16-8399-2_9
Song, D. and Ma, S., ChemMedChem, 2016, vol. 11, pp. 646–659. https://doi.org/10.1002/cmdc.201600041
Dehyaa, H.M., Ali Jabbar, R., Dhurgham, Q.S., and Hayder, K.A., J. Pharm. Neg. Results, 2022, vol. 13, pp. 893–898. https://doi.org/10.47750/pnr.2022.13.S03.137
Ünver, Y., Ünlüer, D., Direkel, S., and Durdağı, S., Turkish J. Chem., 2020, vol. 44, pp. 1164–1176. https://doi.org/10.3906/kim-2004-78
Bertani, G., J. Bacteriol., 1951, vol. 62, pp. 293–300. https://doi.org/10.1128/jb.62.3.293-300.1951
Ergüden, B., Lett. Appl. Microbiol., 2021, vol. 73, pp. 438–445.
Bouyahya, A., Abrini, J., Dakka, N., and Bakri, Y., J. Pharm. Analysis, 2019, vol. 9, pp. 301–311. https://doi.org/10.1016/j.jpha.2019.03.001
Andrade, J.M. and Estevez-Perez, M.G., Analytica Chim. Acta, 2014, vol. 838, pp. 1–12. https://doi.org/10.1016/j.aca.2014.04.057
Funding
This work was supported by regular institutional funding, and no additional grants were obtained.
Author information
Authors and Affiliations
Contributions
The author YÜ synthesized compounds (I) and (II); the authors HBL and BE designed and performed the experiments. The authors YÜ, HBL, and BE wrote the manuscript.
Corresponding author
Ethics declarations
This article does not contain any studies involving patients or animals as test objects.
Informed consent was not required for this article. No conflict of interest was declared by the authors.
Additional information
Publisher's Note. Pleiades Publishing remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Ergüden, B., Lüleci, H.B. & Ünver, Y. Benzothiophene Schiff Bases Disrupt Cytoplasmic Membrane Integrity of Gram-Positive and -Negative Bacteria Cells. Russ J Bioorg Chem 50, 128–137 (2024). https://doi.org/10.1134/S1068162024010096
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1068162024010096