Abstract
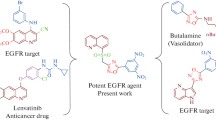
Herein, we synthesized some new indole-1,3,4-oxadiazole based sulfonyl 1,2,3-triazoles via a click chemistry approach and then characterized their structures by NMR, mass, and CHN analysis techniques. Later, the anticancer activity of the synthesized compounds was screened in vitro against different human cancer cell lines like MCF-7 and A-549, and the results were compared with the standard drug erlotinib. Most of the investigated compounds were found to be active against both cancer cell lines, MCF-7, and A-459. Specifically, compounds 2-(((1-(4-chloro-3,5-dimethoxyphenyl)-1H-1,2,3-triazol-4-yl)methyl)sulfonyl)-5-(1-methyl-1H-indol-3-yl)-1,3,4-oxadiazole and 2-(((1-(3,5-dichlorophenyl)-1H-1,2,3-triazol-4-yl)methyl) sulfonyl)-5-(1-methyl-1H-indol-3-yl)-1,3,4-oxadiazole had superior activity against MCF-7, and remarkable activity against A-549. Similarly, the compound 2-(((1-(3,5-dichlorophenyl)-1H-1,2,3-triazol-4-yl)methyl)sulfonyl)-5-(1-methyl-1H-indol-3-yl)-1,3,4-oxadiazole showed more potent activity against EGFR and compound 2-(((1-(4-chloro-3,5-dimethoxyphenyl)-1H-1,2,3-triazol-4-yl)methyl)sulfonyl)-5-(1-methyl-1H-indol-3-yl)-1,3,4-oxadiazole showed equipotent activity against tyrosine kinase EGFR inhibitory activity.


Similar content being viewed by others
REFERENCES
Narsimha, S., Kumar, N.S., Kumaraswamy, B., Vasudeva, R.N., Hussain, A.S., and Srinivasa, R.M., Bioorg. Med. Chem. Lett., 2016, vol. 26, pp. 1639–1644. https://doi.org/10.1016/j.bmcl.2016.01.055
Gibbs, J. B., Science, 2000, vol. 287, pp. 1969–1973. https://doi.org/10.1126/science.287.5460.1969
Arve, L., Voigt, T., and Waldmann, H., QSAR Comb. Sci., 2006, vol. 25, pp. 449–456. https://doi.org/10.1002/qsar.200540213
Rajitha, G., Janardhan, B., Mahendar, P., Ravibabu, V., Sairengpuii, H., Rajitha, B., Sadanandam, A., and Siddhardha, B., Bioorg. Med. Chem. Lett., 2014, vol. 24, pp. 4239–4242. https://doi.org/10.1016/j.bmcl.2014.07.030
Narsimha, S., Sathesh, K.N., Savitha, J.T., Ravinder, M., Srinivasa, R.M., and Vasudeva, R.N., J. Heterocyclic. Chem., 2020, vol. 57, pp. 1655–1665. https://doi.org/10.1002/jhet.3890
Umer, S.M., Solangi, M., Khan, K.M., and Saleem, R.S.Z., Molecules, 2022, vol. 27, p. 7586. https://doi.org/10.3390/molecules27217586
Bahaa, G.M.Y., Mostafa, H.A., Ahmed, H.A., Mohamed, A.A., Hussein, M.I., Ola, I.A.S., Mamdouh, F.A.M., Laurent, T., and Syed, N.A.B., Eur. J. Med. Chem., 2018, vol. 146, pp. 260–273. https://doi.org/10.1016/j.ejmech.2018.01.042
Al-Wahaibi, L.H., Gouda, A.M., Abou-Ghadir, O.F., Salem, O.I.A., Ali, A.T., Farghaly, H.S., Abdelrahman, M.H., Trembleau, L., Abdu-Allah, H.H.M., and Youssif, B.G.M., Bioorg. Chem., 2020, vol. 104, p. 104260. https://doi.org/10.1016/j.bioorg.2020.104260
Zhang, H., Drug Des. Dev. Ther., 2016, vol. 10, pp. 3867–3872. https://doi.org/10.2147/DDDT.S119162
Li, W., Qi, Y.-Y., Wang, Y.-Y., Gan, Y.-Y., Shao, L.-H., Zhang, L.-Q., Tang, Z.-H., Zhu, M., Tang, S.-Y., Wang, Z.-C., and Ouyang, G.-P., J. Heterocycl. Chem., 2020, vol. 57, pp. 2548–2560. https://doi.org/10.1002/jhet.3972
Singh, P.K. and Silakari, O., Bioorg. Chem., 2018, vol. 79, pp. 163–170. https://doi.org/10.1016/j.bioorg.2018.04.001
Anjali, J., Sen, A., and Malla, R.R., Russ. J. Bioorg. Chem., 2021, vol. 47, pp. 670–680. https://doi.org/10.1134/S1068162021030092
Muhammad, A.A., Ramzan, M.S., Aziz-ur-Rehman, Sabahat, Z.S., Syed, A.A.S., Muhammad, A.L., Farman, A.K., and Bushra, M., Russ. J. Bioorg. Chem., 2020, vol. 46, pp. 590–598. https://doi.org/10.1134/S1068162020040020
Dhotre, B.K., Patharia, M.A., Khandebharad, A.U., Raut, S.V., and Pathan, M.A., Russ. J. Bioorg. Chem., 2020, vol. 46, pp. 1110–1116. https://doi.org/10.1134/S1068162020060059
Shalini, B., Partha, P.R., and Jagadish, S., Anticancer. Agents. Med. Chem., 2018, vol. 17, pp. 1869–1883. https://doi.org/10.2174/1871521409666170425092906
Ankur, V., Devender, P., and Kamal, S., Chem. Biol. Drug. Des., 2021, vol. 97, pp. 572–591. https://doi.org/10.1111/cbdd.13795
Vindya, K.G., Ray, U., Mantelingu, K., Sathees, C.R., and Kanchugarakoppal, S.R., Russ. J. Bioorg. Chem., 2020, vol. 46, pp. 837–844. https://doi.org/10.1134/S106816202005009X
Kandukuri, P., Dasari, G., Nukala, S.K., Srinivas, B., and Bhaskar, J., Russ. J. Bioorg. Chem., 2023, vol. 49, pp. 139–146. https://doi.org/10.1134/S1068162023010132
Ramya, S.E., Satheesh, K.N., Ravinder, M., Vasudeva, R.N., and Narsimha, S., Russ. J. Bioorg. Chem., 2021, vol. 47, pp. 896–905. https://doi.org/10.1134/S1068162021040208
Rakesh, S., Narasimha, S.T., Ravinder, M., Vasudeva, R.N., and Narsimha, S., Phosphorus. Sulfur. Silicon. Relat. Elem., 2021, vol. 196, pp. 455–460. https://doi.org/10.1080/10426507.2020.1854257
Ramya, S.E., Thupurani, M.K., Ravinder, M., Gondru, R., and Sirassu, N., Bioorg. Med. Chem. Lett., 2021, vol. 47, p. 128201. https://doi.org/10.1016/j.bmcl.2021.128201
Ramya, S.E., Satheesh, K.N., Narasimha, S.T., Rambabu, P., Rakesh, S., and Sirassu, N., ChemistrySelect, 2023, vol. 8, p. e202204256. https://doi.org/10.1002/slct.202204256
Bennet, I.S., Brooks, G., Broom, N.J.P., Calvert, S.H., Coleman, K., and Francois, I., J. Antibiot., 1991, vol. 44, pp. 969–978. https://doi.org/10.7164/antibiotics.44.969
Stilwell, G.A., Adams, H.G., and Turck, M., Antimicrob. Agents. Chemother., 1975, vol. 8, pp. 751–753. https://doi.org/10.1128/AAC.8.6.75
Soltis, M.J., Yeh, H.J., Cole, K.A., Whittaker, N., Wersto, R.P., and Kohn, E.C., Drug. Metab. Dispos., 1996, vol. 24, pp. 799–806.
Zhi, X., Shi-Jia, Z., and Yi, L., Eur. J. Med. Chem., 2019, vol. 183, p. 111700. https://doi.org/10.1016/j.ejmech.2019.111700
Manoj, K.N., Satheesh, K.N., Narasimha, S.T., Ravinder, M., Thupurani, M.K., and Sirassu, N., J. Mol. Struct., 2022, vol. 1250, p. 131722. https://doi.org/10.1016/j.molstruc.2021.131722
Manoj, K.N, Satheesh, K.N., Narasimha, S.T., Rakesh, S., Ramya, S.E., Pavan, K., and Sirassu, N., J. Mol. Struct., 2022, vol. 1262, p. 132975. https://doi.org/10.1016/j.molstruc.2022.132975
Rajyalakshmi, G., Rama, N.R.A., and Sarangapani, M., Saudi. Pharm. J., 2011, vol. 19, pp. 153–158. https://doi.org/10.1016/j.jsps.2011.03.002
Swathi, C., Sirassu, N., Satheesh, K.N., Bhaskar, P., and Ravinder, M., Russ. J. Bioorg. Chem., 2022, vol. 48, pp. 1314–1321. https://doi.org/10.1134/S1068162022060097
Manmohan, R.D., Suneetha, P., Umesh, C.N., Vinod, D.J., and Siddaiah, V., Russ. J. Bioorg. Chem., 2021, vol. 47, pp. 1028–1033. https://doi.org/10.1134/S1068162021050228
Funding
This work was supported by regular institutional funding, and no additional grants were obtained.
Author information
Authors and Affiliations
Contributions
Author JMRV is research scholar under my supervision who carried out this synthesis and characterization work. Author SKK involved in the supervision of designed chemistry, evaluation of biological activity part, and manuscript writing.
Corresponding author
Ethics declarations
The data that support the findings of this study are available from the corresponding author upon reasonable request. This article does not contain any studies involving animals or human participants performed by any of the authors. Informed consent was not required for this article. No conflict of interest was declared by the authors.
Additional information
Publisher's Note. Pleiades Publishing remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Velidandla, J.M.R., Koppula, S.K. Synthesis of Indole-1,3,4-Oxadiazole Based Sulfonyl 1,2,3-Triazoles as Potent Anticancer and EGFR Inhibitors. Russ J Bioorg Chem 49, 1337–1345 (2023). https://doi.org/10.1134/S1068162023060146
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1068162023060146