Abstract
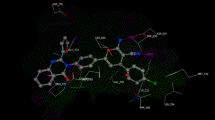
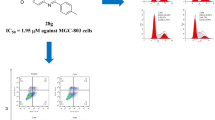
Novel fuoryl quinazoline compounds were synthesized and assessed for anti-cancer efficacy. To varying degrees, all drugs exhibited cytotoxic effect against the test strains. Compound (VIIIc) had the highest IC50 values on MCF7 and HCT116 cell lines, with 25.43 to 23.69 µM, respectively. Compound (VIIIc) shows potent inhibitory activity towards EGFR. Flow cytometric studies revealed that compound (VIIIc) was capable of inducing G2/M arrest with a strong apoptotic effect.





Similar content being viewed by others
REFERENCES
WHO Report on Cancer, 2020, pp. 1–160. https://apps.who.int/iris/rest/bitstreams/1267643/ retrieve.
Zhan, Z., Wang, M., Zhang, C., Zhang, Z., Lu, J., and Wang, F., Chem. Commun., 2015, vol. 51, pp. 9205–9207. https://doi.org/10.1039/C5CC02785C
Madhavi, S., Sreenivasulu, R., Yazala, J.P., and Raju, R.R., Saudi Pharm. J., 2017, vol. 25, pp. 275–279. https://doi.org/10.1016/j.jsps.2016.06.005
Conconi, M.T., Marzaro, G., Urbani, L., Zanusso, I., Di Liddo, R., Castagliuolo, I., Brun, P., Tonus, F., Ferrarese, A., Guiotto, A., and Chilin, A., Eur. J. Med. Chem., 2013, vol. 67, pp. 373–383. https://doi.org/10.1016/j.ejmech.2013.06.057
Alafeefy, A.M., Kadi, A.A., Al-Deeb, O.A., El-Tahir, K.E., and Al-Jaber, N.A., Eur. J. Med. Chem., 2010, vol. 45, pp. 4947–4952. https://doi.org/10.1016/j.ejmech.2010.07.067
Wang, H.J., Wei, C.X., Deng, X.Q., Li, F.L. and Quan, Z.S, Arch. Pharm., 2009, vol. 342, pp. 671–675. https://doi.org/10.1002/ardp.200900119
Georgey, H., Abdel-Gawad, N., and Abbas, S., Molecules, 2008, vol. 13, pp. 2557–2569. https://doi.org/10.3390/molecules13102557
Kasibhatla, S., Baichwal, V., Cai, S.X., Roth, B., Skvortsva, I., Skvortsov, S., Lucas, P., English, N. M., Sirisoma, N., Drewe, J., Pervin, A., Tseng, B., Carlson, R.O., and Pleiman, C.M., Cancer Res., 2007, vol. 67, pp. 5865–5871. https://doi.org/10.1158/0008-5472.CAN-07-0127
Chandrika, P.M., Yakaiah, T., Rao, A.R.R., Narsaiah, B., Reddy, N.C., Srindar, V., and Rao, J.V., Eur. J. Med. Chem., 2008, vol. 43, pp. 846–852. https://doi.org/10.1016/j.ejmech.2007.06.010
Sirisom, N., Pervin, A., Zhan, H., Jiang, S., Willardsen, J. A., Anderson, M.B., Mather, G., Pleiman, C.M., Kasibhatla, S., Tseng, B., Drewe, J., and Cai, S. X., J. Med. Chem., 2009, vol. 52, pp. 2341–2351. https://doi.org/10.1021/jm801315b
Al-Obaid, A. M., Abdel-Hamide, S.G.A., El-Kashef, H. A., Abdel-Aziz, A. A-M., El-Azad, A. S., Al-Khamee, H. A., and El-Subbagh, H. I., Eur. J. Med. Chem., 2009, vol. 44, pp. 2379–2391. https://doi.org/10.1016/j.ejmech.2008.09.015
Font, M., Gonzalez, A., Palop, J.A., and Sanmartin, C., Eur. J. Med. Chem., 2011, vol. 46, pp. 3887–2899. https://doi.org/10.1016/j.ejmech.2011.05.060
Noolvi, M.N., Patel, H.M., Bhardwaj, V., and Chauhan, A., Eur. J. Med. Chem., 2011, vol. 46, pp. 2327–2346. https://doi.org/10.1016/j.ejmech.2011.03.015
Ahmed, M.F. and Belal, A., Res. Chem. Intermed., 2016, vol. 42, pp. 659–671. https://doi.org/10.1007/s11164-015-2048-8
Ahmed, M.F., Belal, A., and Youns, M., Med. Chem. Res., 2015, vol. 24, pp. 2993–3007. https://doi.org/10.1007/s00044-015-1357-1
Ahmed, M.F. and Hashim, A.A.A., Res. Chem. Intermed., 2016, vol. 42, pp. 1777–1789. https://doi.org/10.1007/s11164-015-2117-z
Ahmed, M.F. and Belal, A., Arch. Pharm. Chem. Life Sci., 2015, vol. 348, pp. 487–497. https://doi.org/10.1002/ardp.201400468
Alagarsamy, V., Solomon, V.R., Sheorey, R.V., and Jayakumar, R., Chem. Biol. Drug, Des., 2009, vol, 73, pp. 471–479. https://doi.org/10.1111/j.1747-0285.2009.00794.x
Smits, R.A., Adami, M., Istyastono, E.P., Zuiderveld, O.P., van Dam, C.M.E., de Kanter, F.J.J., Jongejan, A., Coruzzi, G., Leurs, R., and de Esch, I.J., J. Med. Chem., 2010, vol. 53, pp. 2390–2400. https://doi.org/10.1021/jm901379s
Held, F.E., Guryev, A.A., Fröhlich, T., Hampel, F., Kahnt, A., Hutterer, C., Steingruber, M., Bahsi, H., von Bojničić-Kninski, C., Mattes, D.S., and Foertsch, T.C., Nature Comm., 2017, vol. 8, pp. 1–9. https://doi.org/10.1038/ncomms15071
Raghavendra, N.M., Thampi, P., Gurubasavarajaswamy, P.M., and Sriramm D., Chem. Pharm. Bull., 2007, vol. 55, pp. 1615–1619. https://doi.org/10.1248/cpb.55.1615
Das, D. and Hong, J., Eur. J. Med. Chem., 2019, vol. 170, pp. 55–72. https://doi.org/10.1016/j.ejmech.2019.03.004
Ahmed, M. and Magdy, N., Acta Pol. Pharm., 2018, vol. 75, pp. 1321–1328. https://doi.org/10.32383/appdr/89488
Ahmed, M. and Magdy, N., Acta Pol. Pharm., 2018, vol. 75. https://doi.org/10.32383/appdr/89488
He, Y.Y., Huang, J.L., and Chignell, C.F., Oncogene, 2006, vol. 25, pp. 1521–1531. https://doi.org/10.1038/sj.onc.1209184
Bae, S.S., Choi, J.H., Oh, Y.S., Perry, D.K., Ryu, S.H., and Suh, P.G., FEBS Lett., 2001, vol. 491, pp. 16–20. https://doi.org/10.1016/S0014-5793(01)02167-6
Zhang, J., Ren, D., Ma, Y., Wang, W., Wu, H., Tetrahedron, 2014, vol. 70, pp. 5274–5282. https://doi.org/10.1016/j.tet.2014.05.059
Manning, G., Whyte, D.B., Martinez, R., Hunter, T., and Sudarsanam, S., Science, 2002, vol. 298, pp. 1912–1934. https://doi.org/10.1126/science.1075762
Grant, S.K., Cell. Mol. Life Sci., 2009, vol. 66, pp. 1163–1177. https://doi.org/10.1007/s00018-008-8539-7
Wu, Q., Zheng, K., Huang, X., Li, L., and Mei, W., J. Med. Chem., 2018, vol. 61, pp. 10488–10501. https://doi.org/10.1021/acs.jmedchem.8b01018
Ahmed, M.F., Santali, E.Y., and El-Haggar, R., J. Enzyme Inhib. Med. Chem., 2021, vol. 36, pp. 307–318. https://doi.org/10.1080/14756366.2020.1861606
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
ACKNOWLEDGMENT
The researchers would like to acknowledge Deanship of Scientific Research, Taif University for funding this work.
COMPLIANCE WITH ETHICAL STANDARDS
The author declares that he has no conflicts of interest.
This article does not contain any studies involving animals or human participants performed by any of the authors.
Rights and permissions
About this article
Cite this article
Alsantali, R.I. Design, Synthesis, and Anticancer Activity of New Quinazoline Derivatives Containing Acetylhydrazide Moiety as EGFR Inhibitors and Apoptosis Inducers. Russ J Bioorg Chem 49, 645–654 (2023). https://doi.org/10.1134/S1068162023030044
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1068162023030044