Abstract
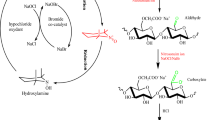
The article presents data on the synthesis and antimicrobial properties of guanidine-containing carboxymethylcellulose derivatives with different physical and chemical characteristics. The regularities of the reaction of nucleophilic substitution of the aldehyde groups of modified Na-carboxymethylcellulose (Na-CMC) by guanidine under different conditions have been studied. It has been found that the maximal replacement of reactive electrophilic groups by nucleophilic reagent depends on the pH value of the medium, molar ratio of guanidine, and the degree of oxidation of cellulose ester. The azomethine derivatives with different content of nitrogen-containing fragments in the polymer chain have been synthesized by varying the reaction conditions and the number of aldehyde groups in oxidized Na-CMC. Chemical reduction of labile azomethine bonds has led to water-soluble derivatives that contain strong amino-bound guanidine groups. The study shows and substantiates the influence of structural parameters (degree of substitution, quantitative guanidine content, pKα values, and nature of counterions) of macromolecular systems on antibacterial and antifungal properties. The developed approach to the synthesis opens up prospects for the creation of antimicrobial derivatives with regulated physical and chemical characteristics and specified biologically active properties.






Similar content being viewed by others
REFERENCES
Dang, X., Liu, P., Yang, M., Deng, H., Shan, Z., and Zhen, W., Production and characterization of dialdehyde cellulose through green and sustainable approach, Cellulose, 2019, vol. 26, pp. 9503–9515. https://doi.org/10.1007/s10570-019-02747-9
Asere, T.G., Mincke, S., and Folens, K., Dialdehyde carboxymethyl cellulose cross-linked chitosan for the recovery of palladium and platinum from aqueous solution, React. Funct. Polym., 2019, vol. 141, pp. 145–154. https://doi.org/10.1016/j.reactfunctpolym.2019.05.008
Akhmedov, O.R., Shomurotov, Sh.A., and Turaev, A.S., Features of the synthesis of dialdehyde derivatives of polysaccharides, Uzb. Khim. Zh., 2013, no. 1, pp. 30–33.
Akhmedov, O.R., Shomurotov, Sh.A., Rakhmanova, G.G., and Turaev, A.S., Synthesis and study of biological activity of sulfamic polysaccharide derivatives, Russ. J. Bioorg. Chem., 2017, no. 7, pp. 718–721. https://doi.org/10.1134/S1068162017070020
Akhmedov, O.R., Shomurotov, Sh.A., Turaev, A.S., and Vaili, A., Synthesis and study of antimicrobial activity of sulphamic pectin derivatives, Chem. Sustainable Developm., 2017, vol. 25, pp. 139–143.
Syutkin, V.N., Nikolaev, A.G., Sazhin, S.A., Popov, V.M., and Zamoryanskii, A.A., Nitrogen-containing derivatives of dialdehyde cellulose. 2. Synthesis of dialdehyde cellulose derivatives with nitrogenous heterocycles, Khim. Rastit. Syr’ya, 2000, no. 1, pp. 5–25.
Medusheva, E.O., Filatov, V.N., Ryl’tsev, V.V., Belov, A.A., and Kulagina, A.S., et al., Dialdehyde cellulose derivatives modified by biologically active substances, Farmatsiya, 2016, no. 1, pp. 52–56.
Gumnikova, V.I., Synthesis of dialdehyde dextran and dialdehyde carboxymethyl cellulose and their chemical transformations, Cand. Sci. (Chem.) Dissertation, Moscow, 2014.
Houben-Weyl, Methods of Organic Chemistry, New York: Georg Thieme, 1998, vol. 2.
Rawlinson, L.B., Ryan, S.M., Mantovani, G., Syrett, J.A., Haddleton, D.M., and Brayden, D.J., Antibacterial effects of poly(2-(dimethylamino ethyl)methacrylate) against selected gram-positive and gram-negative bacteria, Biomacromolecules, 2010, vol. 11, no. 2, pp. 443–453. https://doi.org/10.1021/bm901166y
Tishchenko, E.V., Iozep, A.A., and Ivin, B.A., Reaction of dextran polyaldehyde with aminohydroxypyrimidines, Russ. J. Appl. Chem., 2002, vol. 75, no. 4, pp. 680–682.
Snezhko, V.A., Komar, V.P., Khomyakov, K.P., Virnik, A.D., Zhbankov, R.G., Rozenberg, G.Ya., and Rogovin, Z.A., Synthesis of water-soluble dextran derivatives containing chemically bound antibiotics, Vysokomol. Soedin., 1974, vol. 16, no. 10, pp. 2233–2239.
Sarymsakov, A.A., Nadzhimutdinov, Sh., and Tashpulatov, Yu.T., Chemical transformations in the chains of dialdehydes of cellulose and its esters, Khim. Prirod. Soedin., 1998, no. 2, pp. 212–217.
Shomuratov, Sh.A., Murodov, E.A., and Turaev, A.S., Synthesis and study of a combined anti-tuberculosis drug based on carboxymethylcellulose, Khim. Rastit. Syr’ya, 2006, no. 2, pp. 25–28.
Keshk, S.M.A.S., Ramadan, A.M., and Bondock, S., Physicochemical characterization of novel schiff bases derived from developed bacterial cellulose 2,3-dialdehyde, Carbohydr. Polym., 2015, vol. 127, pp. 246–251. https://doi.org/10.1016/j.carbpol.2015.03.038
Wang, P., He, H., Cai, R., Tao, G., Yang, M., Zuo, H., and Wang, Y., Cross-linking of dialdehyde carboxymethyl cellulose with silk sericin to reinforce sericin film for potential biomedical application, Carbohydr. Polym., 2019, vol. 212, pp. 403–411. https://doi.org/10.1016/j.carbpol.2019.02.069
Tishchenko, E.V., Interaction of amino- and hydroxy(oxo) derivatives of heterocycles with polysaccharides—a new route for the synthesis of biologically active substances, Extended Abstract of Cand. Sci. (Chem.) Dissertation, St. Petersburg, 2003.
Kozlova, Yu.S. and Rogovin, Z.A., Synthesis of new derivatives of cellulose and other polysaccharides, Vysokomol. Soedin., 1960, vol. 2, no. 4, pp. 614–618.
Shatalov, D.O., Development and standardization of quality control methods for branched oligohexamethylene-guanidine hydrochloride, Cand. Sci. (Pharm. Sci.) Dissertation, Moscow, 2015.
Afinogenov, G.E. and Panarin, E.F., Antimikrobnye polimery (Antimicrobial Polymers), St. Petersburg: Gippokrat, 1993.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors state that there is no conflict of interest.
This article does not contain any studies on the use of animals as objects of research.
Additional information
Translated by A. Levina
Rights and permissions
About this article
Cite this article
Akhmedov, O.R., Shomurotov, S.A. & Turaev, A.S. Features of Synthesis and Antimicrobial Properties of Guanidine-Containing Carboxymethylcellulose Derivatives. Russ J Bioorg Chem 48, 1379–1386 (2022). https://doi.org/10.1134/S1068162022070020
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1068162022070020