Abstract—
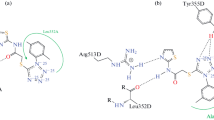
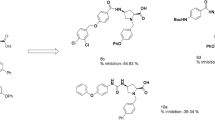
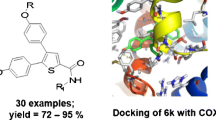
Using the molecular docking approach the simulation of the complex formation of 17 uracil derivatives containing cyclic and acyclic sulfur- and oxygen substituents in the pyrimidine cycle with active centers of cyclooxygenase isoforms (COX) was performed. Of the set tested, two leading compounds were identified, namely, conjugates of 5-hydroxy-1,3,6-trimethyluracil with N-phthalyl-L-amino acids, which can be effective inhibitors of COX isoforms induced during inflammatory processes in the organism with an increased selectivity towards COX-2. The synthesized compounds were evaluated in vivo on the models of inflammation caused by carrageenan, lidocaine, egg white, and formalin. The conjugates of 5-hydroxy-1,3,6-trimethyluracil with N-phthalyl alanine and N-phthalyl methionine displayed pronounced anti-inflammatory activities with the efficacy comparable to that of Ortofen. The isoenzyme-specific inhibition of COX isoforms was assessed and the pronounced anti-inflammatory activity of the synthesized compounds was found.


Similar content being viewed by others
REFERENCES
Grabovskiy, S.A., Murinov, Yu.I., and Kabal’nova, N.N., Curr. Org. Chem., 2012, vol. 16, pp. 2389–2393. https://doi.org/10.2174/138527212803520056
Myshkin, V.A., Ibatullina, R.B., Savlukov, A.I., Bakirov, A.B., and Sergeeva, S.A., Antioksidantnye effekty proizvodnykh pirimidina i benzimidazola pri ostrykh otravleniyakh (Antioxidant effects of pyrimidine and benzimidazole derivatives in acute poisoning). Ufa: OOO PKP “DAR”, 2003.
Myshkin, V.A. and Bakirov, A.B., Eksperimental’naya korrektsiya khimicheskikh porazhenii pecheni proizvodnymi pirimidina (Experimental correction of chemical damage of liver by pyrimidine derivatives). Ufa: OOO PKP “DAR”, 2002.
Kamilov, F.Kh., Lazareva, D.N., and Plechev, V.V., Pirimidiny i ikh primenenie v meditsine (Pyrimidines and their application in medicine). Ufa: Izd. BGMI, 1992.
Lazareva, D.N., Alekhin, E.K., Plechev, V.V., Timerbulatov, V.M., and Plecheva, D.V., Immureg (Immureg). Ufa: Izd-vo BGMU, NPO Bashbiomed, 2004.
Krivonogov, V.P., Tolstikov, G.A., Murinov, Yu.I., Kukovinets, A.G., Shakirova, A.M., Sorokina, G.A., Seleznev, L.G., Vitvitskaya, A.S., Bril’, A.S., Kazakov, V.P., Karavaev, A.D., Zarudii, F.S., Lazareva, D.N., Komissarov, V.D., and Akhunov, I.R., patent RU2000298S1, published 07.09.1993.
Isobe, Y., Tobe, M., Inoue, Y., Isobe, M., Tsuchiya, M., and Hayashi, H., Bioorg. Med. Chem., 2003, vol. 11, pp. 4933–4940. https://doi.org/10.1016/j.bmc.2003.09.012
Curtis-Prior, P., The Eicosanoids, Chester: John Wiley & Sons, 2004.
Dennis, E.A., J. Biol. Chem., 1994, vol. 269, pp. 13057–13060.
Samy, R.P., Gopalakrishnakone, P., and Chow, V.T.K., Bioinformation, 2012, vol. 8, p. 048–057. https://doi.org/10.6026/97320630008048
Flower, R.J. and Blackwell, G.J., Nature, 1979, vol. 278, pp. 456–459. https://doi.org/10.1038/278456a0
Henderson, W.R., Oslund, R.C., Bollinger, J.G., Ye, X., Tien, Y.T., Xue, J., and Gelb, M.H., J. Biol. Chem., 2011, vol. 286, pp. 28049–28055. https://doi.org/10.1074/jbc.M111.235812
Berg, O.G., Gelb, M.H., Tsai, M.D., and Jain, M.K., Chem. Rev., 2001, vol. 101, pp. 2613–2654. https://doi.org/10.1021/cr990139w
Farooqui, A.A., Ong, W.-Y., and Horrocks, L.A., Pharm. Rev., 2006, vol. 58, pp. 591–620. https://doi.org/10.1124/pr.58.3.7
Ong, W.Y., Farooqui, T., Kokotos, G., and Farooqui, A.A., ACS Chem. Neurosci., 2015, vol. 6, p. 814–831. https://doi.org/10.1021/acschemneuro.5b00073
Sapolsky, R.M., J. Neurosci., 1986, vol. 6, pp. 2240–2244. https://doi.org/10.1523/jneurosci.06-08-02240.1986
Mruwat, R., Cohen, Y., and Yedgar, S., Imunnotherapy, 2013, vol. 5, pp. 315–317. https://doi.org/10.2217/imt.13.18
Zarghi, A. and Arfaei, S., Iran. J. Pharm. Res., 2011, vol. 10, pp. 655–683.
Marnett, L.J., Cancer Prev. Res., 2009, vol. 2, pp. 288–290. https://doi.org/10.1158/1940-6207.capr-09-0033
Smith, W.L., DeWitt, D.L., and Garavito, R.M., Annu. Rev. Biochem., 2000, vol. 69, pp. 145–182. https://doi.org/10.1146/annurev.biochem.69.1.145
Greenhough, A., Smartt, H.J.M., Moore, A.E., Ro-berts, H.R., Williams, A.C., Paraskeva, C., and Kaidi, A., Carcinogenesis, 2009, vol. 30, pp. 377–386. https://doi.org/10.1093/carcin/bgp014
Charlier, C. and Michaux, C., Eur. J. Med. Chem., 2003, vol. 38, pp. 645–659. https://doi.org/10.1016/s0223-5234(03)00115-6
Vane, J.R., Bakhle, Y.S., and Botting, R.M., Annu. Rev. Pharmacol. Toxicol., 1998, vol. 38, pp. 97–120. https://doi.org/10.1146/annurev.pharmtox.38.1.97
Cha, Y.I. and DuBois, R.N., Annu. Rev. Med., 2007, vol. 58, pp. 239–252. https://doi.org/10.1146/annurev.med.57.121304.131253
Khairullina, V.R., Gerchikov, A.Ya., Taipov, I.A., Boegel, H., and Zarudii, F.S., Pharm. Chem. J., 2011, vol. 45, pp. 539–546. https://doi.org/10.1007/s11094-011-0675-y
Khairullina, V.R., Taipov, I.A., Gerchikov, A.Ja., and Zarudii, F.S., Pharm. Chem. J., 2012, vol. 46, pp. 553–564. https://doi.org/10.1007/s11094-012-0846-5
Khairullina, V.R., Gerchikov, A.Ya., and Zarudii, F.S., Vestnik Bashkirskogo universiteta (Bashkir University Herald), 2014, vol. 19, pp. 417–422.
Sliwoski, G., Kothiwale, S., Meiler, J., and Lowe, E.W., Jr., Pharmacol. Rev., 2014, vol. 66, pp. 334–395. https://doi.org/10.1124/pr.112.007336
Jansen, J.M., Amaro, R.E., Cornell, W., Tseng, Y.Ja., and Walters, W.P., Future Med. Chem., 2014, vol. 4, pp. 1893–1896. https://doi.org/10.4155/fmc.12.137
Khayrullina, V.R., Gerchikov, A.Ya., Lagunin, A.A., and Zarudii, F.S., Biochemistry (Mosc.), 2015, vol. 80, pp. 74–86. https://doi.org/10.1134/s0006297915010095
Gerchikov, A.Ya., Vasil’ev, M.N., Khayrullina, V.R., Tsypysheva, I.P., and Zarudii, F.S., Pharm. Chem. J., 2015, vol. 49, pp. 582–586. https://doi.org/10.1007/s11094-015-1333-6
Khayrullina, V.R., Taipov, I.A., Gerchikov, A.Ya., Vasil’ev, M.N., Zarudii, F.S., and Boegel, H., Pharm. Chem. J., 2014, vol. 48, pp. 317–322. https://doi.org/10.1007/s11094-014-1102-y
Krivonogov, V.P., Tolstikov, G.A., Murinov, Yu.I., Zarudii, F.S., Lazareva, D.N., Ismagilova, A.F., Volkova, S.S., Sakhautdinova, G.M., Afzaletdinova, N.G., Khisamutdinov, R.A., Spirikhin, L.V., and Krivonogova, I.M., Khim.-farm. Zhurnal (Chemical pharmaceutics journal), 1993, vol. 27, pp. 41–44.
Trinus, F.P., Mokhort, N.A., and Klebanov, B.M., Nesteroidnye protivovospalitel’nye sredstva (Non-steroidal antiinflammatory drugs). Kiev: Zdorov’ya, 1975.
Wang, J., Wolf, R.M., Caldwell, J.M., Kollman, P.A., and Case, D.A., J. Comput. Chem., 2004, vol. 25, pp. 1157–1174. https://doi.org/10.1002/jcc.20035
Dannhardt, G. and Kiefer, W., Eur. J. Med. Chem., 2001, vol. 36, pp. 109–126. https://doi.org/10.1016/s0223-5234(01)01197-7
Khabriev, R.U., Rukovodstvo po eksperimental’nomu (doklinicheskomu) izucheniyu novykh farmakologicheskikh veshchestv, 2-izd., pererab. i dop. (Guide on experimental (preclinical) study of new pharmacological substances, second edition, revised and updated), Moscow: OAO Izd-vo “Meditsina”, 2005.
Funding
The work was supported by the Russian Scientific Foundation, project no. 19-73-20073 and state tasks of Ufa Institute of Chemistry, Russian Academy of Sciences, projects nos. 122031400278-2 and 122031400274-4.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
COMPLIANCE WITH ETHICAL STANDARDS
All the current international, national, and/or institutional principles of animal care were met.
Conflict of Interests
The authors notified about the absence of conflict of interest.
Additional information
Translated by E. Shirokova
Abbreviations: COX, cyclooxygenase; IC50, a concentration when 50% inhibition is achieved; NSAIDs, nonsteroidal anti-inflammatory drugs.
Corresponding author: phone: +7 (987) 476-4900.
Rights and permissions
About this article
Cite this article
Khazimullina, Y.Z., Gimadieva, A.R., Khairullina, V.R. et al. The Synthesis and Anti-inflammatory Studies of New Pyrimidine Derivatives, Inhibitors of Cyclooxygenase Isoforms. Russ J Bioorg Chem 48, 1027–1035 (2022). https://doi.org/10.1134/S1068162022050107
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1068162022050107