Abstract
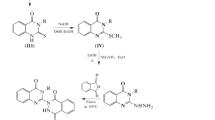
In this study, a novel quinazolinone analogue was designed and synthesized by substituting the thiourea group and phenyl ring at N-3 and C-2 positions of the quinazoline ring, respectively. The prepared analogue was tested for its antibacterial, antitubercular and anti-HIV potencies. The agar dilution method was used to test the antibacterial potency of entire prepared derivatives against various gram-positive and gram-negative microorganism strains. Compound 1-(3-chlorophenyl)-2-methyl-3-(4-oxo-2-methylquinazolin-3(4H)-yl)isothioureas (Xi) shown most potent activity against Klebsiella pneumoniae, Proteus vulgaris, and Staphylococcus epidermidis at 1.6 µg/mL. The compound (Xi) exhibited the antitubercular activity at the minimum microgram of 6.25 µg/mL and anti-HIV activity at 1.17 µg/mL against HIV1 and HIV2. The compound (Xi) offers a potential lead for further optimization and development to new antitubercular and anti-HIV agents. The results obtained from this study confirm that the synthesized and biologically evaluated quinazolines showed promising antimicrobial, antitubercular, and anti-HIV activities and new scaffolds for antimicrobial activity.

Similar content being viewed by others
REFERENCES
World Health Organization, Global Tuberculosis Report 2018, Geneva, Switzerland: World Health Organization, 2018.
Balcha, T.T., Skogmar, S., Sturegård, E., Björkman, P., and Winqvist, N., Glob. Health Action, 2015, vol. 8, pp. 27048–27049. https://doi.org/10.3402/gha.v8.27048
Reid, M.J. and Shah, N.S., Lancet Infect. Dis., 2009, vol. 9, p. 173. https://doi.org/10.1016/S1473-3099(09)70043-X
World Health Organization. Tuberculosis. https://www.who.int/tb/en/. Accessed March 17, 2019.
Alagarsamy, V., Chitra, K., Saravanan, G., Solomon, V.R., Sulthana, M.T., and Narendhar, B., Eur. J. Med. Chem., 2018, vol. 151, pp. 628–685. https://doi.org/10.1016/j.ejmech.2018.03.076
Hameed, A., Al-Rashida, M., Uroos, M., Ali, S.A., Ishtiaq, M., and Khan, K.M., Expert Opin.Ther. Pat., 2018, vol. 28, pp. 281–297. https://doi.org/10.1080/13543776.2018.1432596
Pavan, F.R., Da S Maia, P.I., Leite, S.R., Deflon, V.M., Batista, A.A., Sato, D.N., Franzblau, S.G., and Leite, C.Q., Eur. J. Med. Chem., 2010, vol. 45, pp. 1898–1905. https://doi.org/10.1016/j.ejmech.2010.01.028
Güzel, O., Karali, N., and Salman. A., Bioorg. Med.Chem., 2008, vol. 16, pp. 8976–8987. https://doi.org/10.1016/j.bmc.2008.08.050
Karali, N., Gürsoy, A., Kemirli, F., Shvets, N., Kaynak, F.B., Ozbey, S., Kovalishyn, V., and Dimoglo, A., Bioorg. Med. Chem., 2007, vol. 15, pp. 5888–5904. https://doi.org/10.1016/j.bmc.2007.05.063
Sriram, D., Yogeeswari, P., Thirumurugan, R., and Pavana, R.K., J. Med. Chem., 2006, vol. 49, pp. 3448–3450. https://doi.org/10.1021/jm060339h
Sriram, D., Yogeeswari, P., Dhakla, P., Senthilkumar, P., Banerjee, D., Manjashetty, T.H., Bioorg. Med. Chem. Lett., 2009, vol. 19, pp. 1152–1154. https://doi.org/10.1016/j.bmcl.2008.12.088
Saripinar, E., Güzel, Y., Patat, S., Yildirim, I., Akçamur, Y., and Dimoglo, A.S., Arzneimittelforschung, 1996, vol. 46, pp. 824–828.
Milczarska, B., Foks, H., Sokołowska, J., Janowiec, M., Zwolska, Z., and Rzejczyk, Z., Acta Pol. Pharm., 1999, vol. 56, pp. 121–126.
Turan-Zitouni, G., Ozdemir, A., Kaplancikli, Z.A., Benkli, K., Chevallet, P., and Akalin, G., Eur. J. Med. Chem., 2008, vol. 43, pp. 981–985. https://doi.org/10.1016/j.ejmech.2007.07.001
Pandeya, S.N., Smitha, S., Jyoti, M., and Sridhar, S.K., Acta Pharmaceutica, 2005, vol. 55, pp. 27–46.
Meunier. B., Acc. Chem. Res., 2008, vol. 41, pp. 69–77. https://doi.org/10.1021/ar7000843
Alagarsamy, V., Solomon, V.R., Sheorey, R.V., and Jayakumar. R., Chem. Biol. Drug. Des., 2009, vol. 73, pp. 471–479. https://doi.org/10.1111/j.1747-0285.2009.00794.x
Alagarsamy, V., Appani, R., Sulthana, M.T., Narendar, B., and Solomon, V.R., J. Chilean Chem. Soc., 2016, vol. 61, pp. 2856–2860. https://doi.org/10.4067/S0717-97072016000200002
Sheory, R.V., Thangathiruppathy, A., and Alagarsamy, V., Trop. J. Pharm Res., 2013, vol. 12, pp. 583–589. https://doi.org/10.4314/tjpr.v12i4.21
Alagarsamy V., Solomon V.R., and Dhanabal, K., Bioorg. Med. Chem., 2007, vol. 5, pp. 235–224. https://doi.org/10.1016/j.bmc.2006.09.065
Gobinath, M., Subramanian, N., Alagarsamy, V., Nivedhitha S., and Solomon, V.R., Trop. J. Pharm. Res., 2015, vol. 14, pp. 271–277. https://doi.org/10.4314/tjpr.v14i2.12
Alagarsamy, V., Shankar, D., Murugan, M., Siddiqui, A.A., and Rajesh, R., Arch. Pharm. Chem. Life Sci., 2007, vol. 340, pp. 41–46. https://doi.org/10.1002/ardp.200600189
Alagarsamy, V., Giridhar, R., and Yadav, M.R., J. Pharm. Pharmacol., 2006, vol. 58, pp. 1249–1255. https://doi.org/10.1211/jpp.58.9.0012
Gobinath, M., Subramanian, N., Ruckmani, K., and Alagarsamy, V., Int. J. Drug Discov., 2011, vol. 2, pp. 642–647.
Sriram, D., Yogeeswari, P., Basha, J.S., Radha, D.R., and Nagaraja, V., Bioorg. Med. Chem., 2005, vol. 13, pp. 5774–5778. https://doi.org/10.1016/j.bmc.2005.05.063
Shanmugavelan, P., Nagarajan, S., Sathishkumar, M., Ponnuswamy, A., Yogeeswari, P., and Sriram, D., Bioorg. Med. Chem. Lett., 2011, vol. 21, pp. 7273–7276. https://doi.org/10.1016/j.bmcl.2011.10.048
Kunes, J., Bazant, J., Pour, M., Waisser, K., Slosárek, M., and Janota, J., IL Farmaco, 2000, vol. 55, pp. 725–729. https://doi.org/10.1016/s0014-827x(00)00100-2
Pauwels, R., Clercq, E. De., Desmyter, J., Balzarini, P., Goubabu, P., Herdesijin, P., Verhaughe, H., and Veputle, M. J., Virol. Methods, 1987, vol. 16, pp. 171–185. https://doi.org/10.1016/0166-0934(87)90002-4
Barry, A., Antibiotics in Laboratory Medicine, Baltimore, MD: William Wilkins, 1991, 5th ed., vol. 1.
Pandeya, S.N., Sriram, D., Nath, G., and De Clercq, E., IL Farmaco, 1999, vol. 54, pp. 624–628. https://doi.org/10.1016/s0014-827x(99)00075-0
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
COMPLIANCE WITH ETHICAL STANDARDS
This article does not contain any studies involving human participants performed by any of the authors and does not contain any studies involving animals performed by any of the author.
Conflict of Interests
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Alagarsamy, V., Sulthana, M.T., Chitra, K. et al. Design, Synthesis, and Structure–Activity Relationships of Novel 1-(Substituted)-2-Methyl-3-(4-Oxo-2-Methylquinazolin-3(4H)-yl) Isothioureas for Their Anti-HIV and Antibacterial Activities. Russ J Bioorg Chem 48, 548–556 (2022). https://doi.org/10.1134/S1068162022030025
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1068162022030025