Abstract
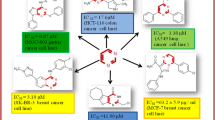
The regioselective synthesis of twelve new benzimidazole-1,2,3-triazole hybrids through the Cu(I)-catalyzed 1,3-dipolar cycloaddition reaction between 1-(3-azidopropyl)-1H-benzo[d]imidazole and several terminal alkynes was described herein. All the synthesized compounds were further evaluated for their in vitro anticancer activity against three human cancer cell lines like PC3, A549, and MCF-7 using etoposide as standard drug. Four compounds named by 1-(3-(4-(3,5-dimethoxyphenyl)-1H-1,2,3-triazol-1-yl)propyl)-1H-benzo[d]imidazole,1-(3-(4-(4-methoxyphenyl)-1H-1,2,3-triazol-1-yl)propyl)-1H benzo[d]imidazole,4-(1-(3-(1H-benzo[d]imidazol-1-yl)propyl)-1H-1,2,3-triazol-4-yl) benzonitrile and 1-(3-(4-(4-nitrophenyl)-1H-1,2,3-triazol-1-yl)propyl)-1H-benzo[d]imidazole were displayed promising activity, in those the compound was shown outstanding activity against PC3, A549, and MCF-7 with IC50 values of 3.01, 3.82, and 2.91 μM respectively.

Similar content being viewed by others
REFERENCES
Neelima, M., Javed Naim, Md., Jahangir Alam, Md., Nawaz, F., Shujauddin, Ah., and Alam, O., Arch. Pharm. Chem. Life Sci., 2017, vol. 350, pp. 1–80. https://doi.org/10.1002/ardp.201700040
Tonelli, M.S., Matteo, T., Bruno, N., Federica, S., Giuseppina, S., Fabio, P., Giuseppe, P., Sabrina, B., Vito, G., Gabriele, B., Sylvain, I., Cristina, L., Roberta, L., and Paolo, C., Bioorg. Med. Chem., 2010, vol. 18. pp. 2937–2953. https://doi.org/10.1016/j.bmc.2010.02.037
Ravishankara, D.K. and Chandrashekara, P.G., Eur. J. Chem., 2012, vol. 3, pp. 359–362. https://doi.org/10.5155/eurjchem.3.3.359-362.607
Love Kumar, S., Narsinghani, T., and Anand, S., Med. Chem. Res., 2012, vol. 21, pp. 4330–4334. https://doi.org/10.1007/s00044-012-9976-2
Deepkumar, J. and Kalpesh, P., Med. Chem. Res., 2014, vol. 23, pp. 1290–1299. https://doi.org/10.1007/s00044-013-0732-z
Galal, S.A., Abdelsamie, A.S., Rodriguez, M.L., Kerwin, S.M., and El Diwani, H.I., Eur. J. Chem., 2010, vol. 1, pp. 67–72. https://doi.org/10.5155/eurjchem.1.2.67-72.1
Kaushik, D., Ahmed Khan, S., and Gita, C., Med. Chem. Res., 2012, vol. 21, pp. 459–467. https://doi.org/10.1007/s00044-011-9552-1
Kolb, H.C. and Sharpless, K.B., Drug Discov. Today, 2009, vol. 8, pp. 1128–1137. https://doi.org/10.1016/S1359-6446(03)02933-7
Agalave, S.G., Maujan, S.R., and Pore, V.S., Chem. Asian J., 2011, vol. 6, pp. 2696–2718. https://doi.org/10.1080/00397911.2019.1616301
Rostovtsev, V.V. and Sharpless, K.B., Angew. Chem. Int. Ed., 2002, vol. 41, pp. 2596–2599. https://doi.org/10.1002/1521-3757 (20020715)114:14<2708::AID ANGE2708>3.0.CO;2-0
Saqlain, H., Mohammad, S.A., Hinna, H., Syed, S., Abhijeet, D., Firasat, H., Perwez, A., Sadiq, U., Pasha, M.A.Q., Sameena, B., Syed, N., Yakub, A., and Chetna, K., Eur. J. Med. Chem., 2014, vol. 81. pp. 204–217. https://doi.org/10.1016/j.ejmech.2014.05.012
Jin-Mei, X., En, Z., Xiao-Jing, S., Yan-Cha, W., Bin, Y., Wei-Wei, J., Ya-Zhuo, G., and Hong-Min, L., Eur. J. Med. Chem., 2014, vol. 80, pp. 593–604. https://doi.org/10.1016/j.ejmech.2014.03.022
Narasimha Swamy Thirukovela, Shravan Kumar, K., Ranjith Kumar, K., Suresh, P., Mohan Rao, G., Chandra Sekhar, V., and Ravinder, V., Med. Chem. Res., 2017, vol. 26, pp. 2190–2195. https://doi.org/10.1007/s00044-017-1926-6
Reddy, D.M., Srinivas, J., Chashoo, G., Saxena, A.K., and Kumar, H.M.S., Eur. J. Med. Chem., 2011, vol. 46, pp. 1983–1991. https://doi.org/10.1016/j.ejmech.2011.02.016
Odlo, K., Hentzen, J., Chabert, J.F.D., Ducki, S., and Gani, I., Bioorg.Med. Chem., 2008, vol. 16, pp. 4829–4838. https://doi.org/10.1016/j.bmc.2008.03.049
Singh, P., Raj, R., Kumar, V., Mahajan, M.P., Bedi, P.M.S., Kaur, T., and Saxena, A.K., Eur. J. Med. Chem., 2012, vol. 47, pp. 594–600. https://doi.org/10.1016/j.ejmech.2011. 10.033
Stefely, J.A., Palchaudhuri, R., Miller, P.A., Peterson, R.J., Moraski, G.C., Hergenrother, P.J., and Miller, M.J., J. Med. Chem., 2010, vol. 53, pp. 3389–3395. https://doi.org/10.1021/jm1000979
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
COMPLIANCE WITH ETHICAL STANDARDS
This article does not contain any studies involving animals or human participants performed by any of the authors.
Conflict of Interests
The authors declare that they have no conflict of interest
Rights and permissions
About this article
Cite this article
Manmohan Reddy Depa, Potla, S., Narkhede, U.C. et al. Cu(I)-Promoted Regioselective Synthesis of Some New Benzimidazole-1,2,3-Triazole Frameworks as In Vitro Anticancer Agents. Russ J Bioorg Chem 47, 1028–1033 (2021). https://doi.org/10.1134/S1068162021050228
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1068162021050228