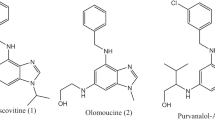
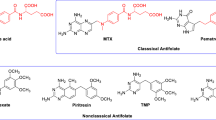
Abstract
In search of the best anticancer agents, a series of novel fused [1,2,3]triazolo[4',5':3,4] pyrrolo[2,1-f]purine derivatives in one vessel was synthesized using 8-bromo-1,3-dimethyl-7-(prop-2-yn-1-yl)-1H-purine-2,6(3H,7H)-dione and various arylazides. The newly synthesized derivatives were evaluated for their in vitro anti-proliferative activity against four human cancer cell lines (MCF-7, HeLa, A-549 and U-87MG). 3-(3,5-dichlorophenyl)-5,7-dimethyl-7,10-dihydro-[1,2,3]triazolo[4',5':3,4]pyrrolo[2,1-f]purine-6,8(3H,5H)-dione and 5,7-dimethyl-3-(4-nitrophenyl)-3,10-dihydro-[1,2,3]triazolo[4',5':3.4]pyrrolo[2,1-f]purine-6,8 (5H,7H)-dione are showed stronger activity against MCF-7 and A-549 with IC50 values ranging from 11.5 ± 0.64 to 15.3 ± 0.81 μM, which are comparable to the standard drug doxorubicin. Molecular docking studies have also been conducted to complement the experimental results.




Similar content being viewed by others
REFERENCES
Nivedita, S., Ashwinee, K.S., Thakur, M.S., and Sanjukta, P., Heliyon, 2018, vol. 4, no. 10, e00829. https://doi.org/10.1016/j.heliyon.2018.e00829
Allwood, M.B., Cannan, B., Van Aalten, D.M.F., and Eggleston, I.M., Tetrahedron., 2007, vol. 63, pp. 12294–12302. https://doi.org/10.1016/j.tet.2007.09.067
Hayallah, A. M., Elgaher, W.A., Salem, O. I., and Abdel Alim, A.A.M., Arch. Pharm. Res., 2011, vol. 34, pp. 3–21. https://doi.org/10.1007/s12272-011-0101-8
Matthias, E., Elke, L., Michael, M., Moh, T., Leo, T., Herbert, N., Waldemar, P., Brian, G., Ralf, L., Peter, S., Holger, F., and Frank, H., J. Med. Chem., 2007, vol. 50, pp. 6450–6453. https://doi.org/10.1021/jm701280z
Gang, L., Yi, H., Baokun, Y., Jin, W., Qian, J., Ziyun, L., Zhufang, S., and Haihong, H., Eur. J. Med. Chem., 2016, vol. 124, pp. 103–116. https://doi.org/10.1016/j.ejmech.2016.08.023
Yiwen, H., Xiaoqing, H., Taizhi, W., and Fuli, Z., Molecules, 2016, vol. 21, pp.1041. https://doi.org/10.3390/molecules21081041
Yan, R., Heying, P., Mingfeng, S., and Lijuan, C., Chem. Biol. Drug. Des., 2016, vol. 87, pp. 290–295. https://doi.org/10.1111/cbdd.12663
Sirassu, N., Kumara, S.B., Ravinder, M., Reddy, Y.N., and Vasudeva, R.N., J. Chem. Sci., 2020, vol. 132, pp. 59. https://doi.org/10.1007/s12039-020-1760-0
Glennon, R.A., Gaines, J.J., and Rogers, M.E., J. Med. Chem., 1981, vol. 24, pp. 766–769. https://doi.org/10.1021/jm00138a027
Wong, E.H.-A. and Ooi, S.-O., Biochem. Pharmacol., 1985, vol. 34, pp. 2891–2896. https://doi.org/10.1016/0006-2952(85)90012-7
Haginaka, J., Wakai, J., Yasuda, H., and Kimura, Y., J. Chromatogr. B Biomed. Sci. Appl., 1990, vol. 529, pp. 455–461. https://doi.org/10.1016/s0378-4347(00)83854-2
Constantin, S., Lupascu, F.G., Apotrosoaei, M., Vasincu, I.M., Lupascu, D., and Buron, F., Chem. Cent. J., 2017, vol.11, p. 12. https://doi.org/10.1186/s13065-017-0241-0
Dai, Z.-K., Liu, Y.-W., Hsu, J.-H., Yeh, J.-L., Chen, I.-J., and Wu, J.-R., Int. J. Biol. Sci., 2015, vol.11, pp. 633–642. https://doi.org/10.7150/ijbs.11127
Slattery, M.L. and West, D.W., Cancer Causes Control, 1993, vol. 4, pp. 559–563. https://doi.org/10.1007/BF00052432
Barcz, E., Sommer, E., Janik, P., Marianowski, L., and Skopinska-Rózewska, E., Oncol. Rep., 2000, vol. 7, pp. 1285–1291. https://doi.org/10.3892/or.7.6.1285
Kakuyama, A., and Sadzuka, Y., Curr. Drug Metabol., 2001, vol. 2, pp. 379–395. https://doi.org/10.2174/1389200013338270
Zhang, Y., Yu, J., Zhang, L., Cai, J., Cai, D., and Lv, C., Tumor Biol., 2016, vol. 37, pp. 2703–2708. https://doi.org/10.1007/s13277-015-4106-7
Agalave, S.G., Maujan, S.R., and Pore, V.S., Chem. Asian J., 2011, vol. 6, pp. 2696–2718. https://doi.org/10.1002/asia.201100432
El-Sagheer, A.H. and Brown, T., Acc. Chem. Res., 2012, vol. 45, pp. 1258–1267. https://doi.org/10.1021/ar200321n
Thirumurugan, P., Matosiuk, D., and Jozwiak, K., Chem. Rev., 2013, vol. 113, pp. 4905–4979. https://doi.org/10.1021/cr200409f
Bonandi, E., Christodoulou, M.S., Fumagalli, G., Perdicchia, D., Rastelli, G., and Passarella, D., Drug. Discov. Today., 2017, 2ol. 2, pp. 1572–1581. https://doi.org/10.1016/j.drudis.2017.05.014
Narsimha, S., Kumara, S.B., Satheesh, K. N., Ramesh, G., Yellu, N.R., and Vasudeva, R. N., RSC Adv., 2016, vol. 6, pp. 74332–74339. https://doi.org/10.1039/C6RA12285J
Narsimha, S., Kumara, S.B., Yellu, N.R., Vasudeva, R.N., Chem. Heterocycl. Compd., 2018, vol. 54, pp. 1161–1167. https://doi.org/10.1007/s10593-019-02408-6
Senwar, K.R., Sharma, P., Reddy, T.S., Jeengar, M.K., Nayak, V.L., Naidu, V.G., Kamal, A., and Shankaraiah, N., Eur. J. Med. Chem., 2015, vol. 102, pp. 413–424. https://doi.org/10.1016/j.ejmech.2015.08.017
Sheng-Jiao, Y., Yong-Jiang, L., Yu-Lan, C., Lin, L., and Jun, L., Bioorg. Med. Chem. Lett., 2010, vol. 20, pp. 5225–5228. https://doi.org/10.1016/j.bmcl.2010.06.141
Chen, C.Y., Yang, C.H., Hu, W.P., Vandavasi, J.K., Chung, M.I., and Wang, J.J., RSC Adv., 2013, vol. 3, pp. 2710–2719. https://doi.org/10.1039/C2RA22799A
Srivari, C., Mallikanti, S., Abhishek, K., Chada, R. R., Suman, K. M., Chityal, G. K., and Sridhar, B., Tetrahedron Lett., 2011, vol. 52, pp. 806–808. https://doi.org/10.1016/j.tetlet.2010.12.040
Hsin-Yu, H., Wen-Chun, L., Gopal, C.S., WanPing, H., Jium-Jia, L., Tong-Rong, T., Yu-Wei, C., Kung-Kai, K., Chung-Yu, C., and Jeh-Jeng, W., J. Med. Chem., 2013, vol. 56, pp. 5422–5435. https://doi.org/10.1021/jm400394s
Ramesh, B.H., Ravinder, M., Narsimha, S., Indian J. Heterocycl. Chem., 2019, vol. 29, pp. 389–395.
Narsimha, S., Kumar, N.S., Kumaraswamy, B., Vasudeva, R.N., Hussain, A.S., and Srinivasa, R.M., Bioorg. Med. Chem. Lett., 2016, vol. 26, pp.1639–1644. https://doi.org/10.1016/j.bmcl.2016.01.055
Swamy, B.K., Narsimha, S., Kumar, T.R., Reddy, Y.N., and Reddy, N.V., Chem. Select., 2017, vol. 2, pp. 9595–9598. https://doi.org/10.1002/slct.201701902
Narsimha, S., Battula, K.S., and Nagavelli, V.R., Syn. Commun., 2018, vol. 48, pp. 1220–1226. https://doi.org/10.1080/00397911.2018.1440315
Swamy, B.K., Narsimha, S., Kumar, T.R., Reddy, Y.N., and Reddy, N.V., Chem. Select., 2017, vol. 2, pp. 4001–4005. https://doi.org/10.1002/slct.201700524
Ramesh, B.H., Narsimha, S., Ravinder, M., Janapatla, U.R., Indian J. Heterocycl. Chem., 2020, vol. 30, pp. 233–238.
Mosmann, T., J. Immunol. Methods, 1983, vol. 65, pp. 55–63. https://doi.org/10.1016/0022-1759(83)90303-4
Botta, M., Armaroli, S., Castagnolo, D., Fontana, G., Pera, P., and Bombardelli, E., Bioorg. Med. Chem. Lett., 2007, vol. 17, pp. 1579–1583. https://doi.org/10.1016/j.bmcl.2006.12.101
Park, J.H. and Lemmon, M.A., Biochem. J., 2012, vol. 448, pp. 417–423. https://doi.org/10.1042/BJ20121513
Sebastian, J., Richards, R.G., Walker, M. P., Wiesen, J.F., Werb, Z., Derynck, R., Hom,Y. K., Cunha, G.R., and DiAugustine, R. P., Cell Grow. Diff., 1998, vol. 9, pp. 777–785.
McBryan, J., Howlin, J., Napoletano, S., and Martin, F., J. Mammary Gland Biol. Neoplasia, 2008, vol. 13, pp.159–167. https://doi.org/10.1007/s10911-008-9075-7
Walker, F., Abramowitz, L., Benabderrahmane, D., Duval, X., Descatoire, V., Henin, D., Lehy, T., and Aparicio, T., Hum. Path., 2009, vol. 40, pp. 1517–1527. https://doi.org/10.1016/j.humpath.2009.05.010
Roskoski, R. Jr., Pharmacol Res., 2014, vol. 79, pp. 34–74. https://doi.org/10.1016/j.phrs.2013.11.002
ACKNOWLEDGMENTS
The authors are thankful to the head, Department of Bio-Technology, Kakatiya University, and Warangal for providing data of biological activity.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
COMPLIANCE WITH ETHICAL STANDARDS
No animals were involved in this work. No human subjects were involved in this work.
Conflict of Interests
No conflict of interest was declared by the authors.
Supplementary Information
Rights and permissions
About this article
Cite this article
E. Ramya Sucharitha, Kumar, N.S., Ravinder, M. et al. Synthesis and Biological Evaluation of Novel Fused [1,2,3]Triazolo[4',5':3,4] pyrrolo[2,1-f]purines as Potent Anti-Proliferative Agents. Russ J Bioorg Chem 47, 896–905 (2021). https://doi.org/10.1134/S1068162021040208
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1068162021040208