Abstract
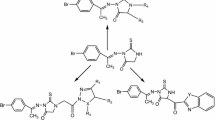
New compounds based on Furanone derivatives were synthesized by reaction of 3-(4-nitrobezylidine)-5-phenylfuran-2(3H)-one with various reagents as malononitrile then D-glucose, thiosemicarbazide then D-glucose, ethyl acetoacetate, acetyl acetone, ethyl cyanoacetate, hydrazine hydrate/acetic acid, thiourea, o-phenylene diamine, hydrazine hydrate/ethanol then 2-naphthalene thionylchloride and/or thiourea. Also, 3-(4-nitrobezylidine)-5-phenylfuran-2(3H)-one was reacted with benzyl amine to afford the 1,3-dihydro-2H-pyrrol-2-one derivative which was reacted with hydroxyl amine and/or phenyl hydrazine to afford compounds the oxazole and/or pyrazole derivatives respectively. Finely, 6-(4-nitrophenyl)-5-(2-oxo-2-phenylethyl)-2-thioxotetrahydro-pyrimidin-4(1H)-one was allowed to react with 2-oxo-N-phenylpropanehydrazonoyl chloride yielding [1,2,4]triazolo[4,3-a]pyrimidin-7(1H)-one derivative. The anti-cancer activity of some of some of the new synthesized compounds towards breast carcinoma cells (MCF-7) was evaluated that demonstrated good to moderate results. Also, we had evaluated the cytotoxicity of the new tested compounds against the normal cells (MRC-5) which showed low toxicity on them.









Similar content being viewed by others
REFERENCES
Vane, J.R., Bakhle, Y.S., and Botting, R.M., Annu. Rev. Pharmacol. Toxicol., 1998, vol. 38, pp. 97–120. https://doi.org/10.1146/annurev.pharmtox.38.1.97
Botting, J.H., Drugs Today, 1999, vol. 35, pp. 225–235.
Kurumbail, R.G., Stevens, A.M., Gierse, J.K., McDonald, J.J., Stegeman, R.A., Pak, J.Y., Gildehaus, D., Iyashiro, J.M., Penning, T.D., Seibert, K., Isakson, P., and Stalling, W.C., Nature, 1996, vol. 384, pp. 644–648. https://doi.org/10.1038/384644a0
Simmons, D. L., Botting, R. M., and Hla, T., Pharmacol. Rev., 2004, vol. 56, pp. 387–437. https://doi.org/10.1124/pr.56.3.3
Kargman, S.L., O’Neill, G.P., Vickers, P.J., Evans, J.F., Mancini, J.A., and Jothy, S., Cancer Res., 1995, vol. 55, pp. 2556–2559.
Gupta, A.K., Gupta, R.A., Soni, L. K., and Kaskhedikar, S.G., Eur. J. Med. Chem., 2008, vol. 43, pp. 1297–1303. https://doi.org/10.1016/j.ejmech.2007.06.022
Singh, P., Mittal, A., Kaur, S., and Kumar, S., Eur. J. Med. Chem., 2008, vol. 43, pp. 2792–2799. https://doi.org/10.1016/j.ejmech.2007.12.017
Yao, X.J., Panaye, A., Doucet, J.P., Zhang, R.S., Chen, H.F., Liu, M.C., Hu, Z.D., and Fan, B.T., J. Chem. Inf. Comput. Sci., 2004, vol. 44, pp. 1257–1266. https://doi.org/10.1021/ci049965i
Ravichandran, V., Mourya, V.K., and Agrawal, R.K., Arkivoc, 2007, vol. 14, pp. 204–212. https://doi.org/10.3998/ark.5550190.0008.e07
Gupta, S.P., Babu, M.S., Garg, R., and Sowmya, S. J., Enzyme Inhib., 1998, vol. 13, pp. 399–407. https://doi.org/10.3109/14756369809020545
Hansch, C. and Fujita, T., J. Am. Chem. Soc., 1964, vol. 86, pp. 1616–1626. https://doi.org/10.1021/ja01062a035
Bihelovic, F. and Saicic, R.N., Angew. Chem. Int. Ed., 2012, vol. 51, pp.5687–5691. https://doi.org/10.1002/anie.201108223
Trost, B.M., Burns, A.C., Bartlett, M.J., Tautz, T., and Weiss, A.H., J. Am. Chem. Soc., 2012, vol. 134, pp. 1474–1477. https://doi.org/10.1021/ja210986f
Li, J., Yang, P., Yao, M., Deng, J., and Li, A., J. Am. Chem. Soc., 2014, vol. 136, pp. 16 477–16 480. doi.org/10.1021/ja5092563
You, L., Liang, X.-T., Xu, L.-M., Wang, Y.-F., Zhang, J.-J., Su, Q., Li, Y.-H., Zhang, B., Yang, S.-L., and Chen, J.-H., J. Am. Chem. Soc., 2015, vol. 137, pp. 10 120–10 123. https://doi.org/10.1021/jacs.5b06480
Trost, B.M. and Hitce, J., J. Am. Chem. Soc., 2009, vol. 131, pp. 4572–4573. https://doi.org/10.1021/ja809723u
Ube, H., Shimada, N., and Terada, M., Angew. Chem. Int. Ed., 2010, vol. 49, pp. 1858–1861. https://doi.org/10.1002/anie.201100646
Jiang, B., Feng, B.-M., Wang, S.-L., Tu, S.-J., and Li, G.-G., Chem. Eur. J., 2012, vol. 18, pp. 9823–9826. https://doi.org/10.1002/chem.201201862
Yavorskyy, A., Shvydkiv, O., Hoffmann, N., Nolan, K., and Oelgemoller, M., Org. Lett., 2012, vol. 14, pp. 4342–4345. https://doi.org/10.1021/ol301773r
Moller, T., Wonneberger, P., Kretzschmar, N., and Hey-Hawkins, E., Chem. Commun., 2014, vol. 50, pp. 5826–5828.
Hoogenboom, J., Lutz, M., Zuilhof, H., and Wennekes, T., J. Org. Chem., 2016, vol. 81, pp. 8826–8836 https://doi.org/10.1021/acs.joc.6b01515
Lattmann, E., Dunn, S., Niamsanit, S., and Sattayasai, N., Bioorg. Med. Chem. Lett., 2005, vol. 15, pp. 919–921. https://doi.org/10.1016/j.bmcl.2004.12.051
Semenova, M.N., Kiselyov, A.S., Tsyganov, D.V., Konyushkin, L.D., Firgang, S.I., Semenov, R.V., Malyshev, O.R., Raihstat, M.M., Fuchs, F., Stielow, A., Lantow, M., Philchenkov, A.A., Zavelevich, M.P., Zefirov, N.S., Kuznetsov, S.A., and Semenov, V.V., J. Med. Chem., 2011, vol. 54, pp. 7138–7149. https://doi.org/10.1021/jm200737s
Xiao, Z.-P., Ma, T.-W., Liao, M.-L., Feng, Y.-T., Peng, X.-C., Li, J.-L., Li, Z.-P., Wu, Y., Luo, Q., Deng, Y., Liang, X., and Zhu, H.-L., Eur. J. Med. Chem., 2011, vol. 46, pp. 4904–4914. doi.org/10.1016/j.ejmech.2011.07.047
Kumar, A., Kumar, V., Alegria, A.E., and Malhotra, S.V., Curr. Med., Chem. 2011, vol. 18, pp. 3853–3870. https://doi.org/10.2174/092986711803414331
Kamal, A., Srinivasa Reddy, T., Polepalli, S., Paidakula, S., Srinivasulu, V., Ganga Reddy, V., Jain, N., and Shankaraiah, N., Bioorg. Med. Chem. Lett., 2014, vol. 24, pp. 3356–3360. doi.org/10.1016/j.bmcl.2014.05.096
Dai, Y., Zhou, G.-X., Kurihara, H., Ye, W.-C., and Yao, X.-S., J. Nat. Prod., 2006, vol. 69, pp. 1022–1024. https://doi.org/10.1021/np0600853
Kim, K.H., Choi, S.U., Ha, S.K., Kim, S.Y., and Lee, K.R., J. Nat. Prod., 2009, vol. 72, pp. 2061–2064. https://doi.org/10.1021/np900460j
Newson, H.L., Wild, D.A., Yeung, S.Y., Skelton, B.W., Flematti, G.R., Allan, J.E., and Piggott, M.J., J. Org. Chem., 2016, vol. 81, pp. 3127–3135. https://doi.org/10.1021/acs.joc.5b02861
Asselah, T.J., Hepatol., 2011, vol. 54, pp. 1069–1072. https://doi.org/10.1016/j.jhep.2010.11.033
Singh, C., Verma, V.P., Hassam, M., Singh, A.S., Naikade, N.K., and Puri, S.K., J. Med. Chem., 2014, vol. 57, pp. 2489–2497. https://doi.org/10.1021/jm401774f
Ghisaidoobe, A.T., van den Berg, R.J.B.H.N., Butt, S.S., Strijland, A., Donker-Koopman, W.E., Scheij, S., van den Nieuwendijk, A.M.C.H., Koomen, G.-J., van Loevezijn, A., Leemhuis, M., Wennekes, T., van der Stelt, M., van der Marel, G.A., van Boeckel, C.A.A., Aerts, J.M.F.G., and Overkleeft, H.S., J. Med.Chem. 2014, vol. 57, pp. 9096–9104. https://doi.org/10.1021/jm501181z
Ghosh, A.K., Yu, X.F., Osswald, H.L., Agniswamy, J., Wang, Y.F., Amano, M., Weber, I.T., and Mitsuya, H., J. Med. Chem., 2015, vol. 58, pp. 5334–5343. https://doi.org/10.1021/acs.jmedchem.5b00676
Guglielmo, S., Lazzarato, L., Contino, M., Perrone, M.G., Chegaev, K., Carrieri, A., Fruttero, R., Colabufo, N.A., and Gasco, A., J Med. Chem., 2016, vol. 59, pp. 6729–6738. https://doi.org/10.1021/acs.jmedchem.6b00252
Gao, M., Nettles, R.E., Belema, M., Snyder, L.B., Nguyen, V.N., Fridell, R.A., Serrano-Wu, M.H., Langley, D.R., Sun, J.H., O’Boyle, D.R., Lemm, J.A., Wang, C.F., Knipe, J.O., Chien, C., Colonno, R.J., Grasela, D.M., Meanwell, N.A., and Hamann, L.G., Nature, 2010, vol. 465, pp. 96–100.
Bae, I.H., Choi, J.K., Chough, C., Keum, S.J., Kim, H., Jang, S.K., and Kim, B.M., ACS Med. Chem. Lett., 2014, vol. 5, pp. 255–258. https://doi.org/10.1021/ml4003293
Bae, I.H., Kim, H.S., You, Y., Choug, C., Choe, W., Seon, M.K., Lee, S.G., Keum, G., Jang, S.K., Kim, B.M., Eur. J. Med. Chem., 2015, vol. 101, pp. 163–178. https://doi.org/10.1016/j.ejmech.2015.06.033
Palmer, B.D., Thompson, A.M., Sutherland, H.S., Blaser, A., Kmentova, I., Franzblau, S.G., Wan, B., Wang, Y., Ma, Z., and Denny, W.A., J. Med. Chem., 2010, vol. 53, pp. 282–294. https://doi.org/10.1021/jm901207n
Hughes, A.D., Chen, Y., Hegde, S.S., Jasper, J.R., Jaw-Tsai, S., Lee, T.W., McNamara, A., Pulido-Rios, M.T., Steinfeld, T., and Mammen, M., J. Med. Chem., 2015, vol. 58, pp. 2609–2622. https://doi.org/10.1021/jm501915g
Lai, M.-J., Lee, H.-Y., Chuang, H.-Y., Chang, L.-H., Tsai, A.-C., Chen, M.-C., Huang, H.-L., Wu, Y.-W., Teng, C.-M., Pan, S.-L., Liu, Y.-M., Mehndiratta, S., and Liou, J.-P., J. Med. Chem., 2015, vol. 58, pp. 6549–6558. https://doi.org/10.1021/acs.jmedchem.5b00659
Niece, K.L., Hartgerink, J.D., Donners, J.J.J.M., and Stupp, S.I., J., Am. Chem. Soc., 2003, vol. 125, pp. 7146–7147. https://doi.org/10.1021/ja028215r
Horgan, C.C., Rodriguez, A.L., Li, R., Bruggeman, K.F., Stupka, N., Raynes, J.K., Day, L., White, J. W., Williams, R. J., and Nisbet, D.R., Acta Biomater., 2016, vol. 38, p. 11. https://doi.org/10.1016/j.actbio.2016.04.038
El-Sayed W.A., Nassar I.F., and Abdel-Rahman A.A., J. Heterocycl. Chem., 2011, vol. 48, pp. 135–143. https://doi.org/10.1002/jhet.522
Chevallier, F., Halauko, Y.S., Pecceu, C., Nassar, I.F., Dam, T.U., Roisnel, T., Matulis, V. E., Ivashkevich, O. A., and Mongin, F., Org. Biomol. Chem., 2011, vol. 9, pp. 4671–4684. https://doi.org/10.1039/c1ob05267e
Nassar, I.F. and Assaly, S.A.-E., Pharma Chem., 2011, vol. 3, pp. 229–238.
Abdel Rahman, A.A., Nassar, I.F., El Katan, I.M.H., Aly, A.A., and Behalo, M.S., Pharma Chem., 2013, vol. 5, pp. 210–217.
Nassar, I.F., J. Heterocycl. Chem., 2013, vol. 50, pp. 129–134. https://doi.org/10.1002/jhet.1022
Nassar, I.F., Atta-Allah, S.R., and Elgazwy, A.S.H., J. Enzym. Inhib. Med. Chem., 2015, vol. 30, p. 396. https://doi.org/10.3109/14756366.2014.940936
Abou El Saou,d, Y.M.H., El Gazwy, A.-S.S.H., Nassar, I.F., Ismail, N.S.M., and Abdel Sattar, N.A., WO Patent no. 2015127941 A1, 2015.
Abu-Dief, A.M., Nassar, I.F., and Elsayed, W.H., Appl. Organometal. Chem., 2016, vol. 30, pp. 917–923. https://doi.org/10.1002/aoc.3521
Nassar, I.F., El Farargy, A.F., Abdelrazek, F.M., and Ismail, N.S.M., Nucleosides Nucleotides Nucleic Acids, 2017, vol. 36, pp. 275–291. https://doi.org/10.1080/15257770.2016.1276290
Nassar, I.F., El Farargy, A.F., and Abdelrazek, F.M., J. Heterocyclic Chem., 2018, vol. 55, p. 1709. https://doi.org/10.1002/jhet.3208
Nassar, I.F., Att-Allah, S.R., and Hemdan, M.M., Phosphorous Sulphur Silicon, 2018, vol. 193, pp. 630–636. https://doi.org/10.1080/10426507.2018.1487435
Nassar, I. F., El-Sayed, W. A., Ragab, T.I.M., Shalaby, A.S.G., and Mehany, A., Mini Rev. Med. Chem., 2019, vol. 9, pp. 395–409. https://doi.org/10.2174/1389557518666180820125210
El-Shehry, M.F., Abu-Zied, K.M., Ewies, E.F., Awad, S.M., and Mohram, M.E., Pharma Chem., 2013, vol. 5, pp. 318–326.
Eweiss, N.F. and Osman, A., J. Heterocycl. Chem., 1980, vol. 17, pp. 1713–1717. https://doi.org/10.1002/jhet.5570170814
Mosmann, T.J., Immunol. Methods, 1983, vol. 65, pp. 55–63. https://doi.org/10.1016/0022-1759(83)90303-4
Gomha, S.M., Riyadh, S.M., Mahmmoud, E.A., and Elaasser, M.M., Heterocycles, 2015, vol. 91, pp. 1227–1243. https://doi.org/10.3987/COM-15-13210
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
COMPLIANCE WITH ETHICAL STANDARDS
This article does not contain any studies involving human participants performed by any of the authors and does not contain any studies involving animals performed by any of the authors.
Conflict of Interests
The authors declare no conflict of interests, financial or otherwise.
Rights and permissions
About this article
Cite this article
Lashin, W.H., Nassar, I.F., El Farargy, A.F. et al. Synthesis of New Furanone Derivatives with Potent Anticancer Activity. Russ J Bioorg Chem 46, 1074–1086 (2020). https://doi.org/10.1134/S1068162020060163
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1068162020060163