Abstract
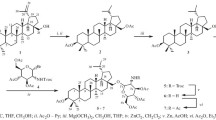
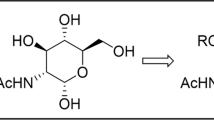
Conjugates of 3,4,6-tri-О-acetyl-N-acetylglucosamine and tetraacetyl glucopyranose with alkyl phosphates were synthesized. The dependence of their antibacterial and antituberculosis activities on the length of the alkyl substituent at the phosphate group was found. The conjugates with a decyl substituent exhibited in vitro the highest antituberculosis activity against Mycobacterium tuberculosis H37Rv (MIC 3 µg/mL) but the weakest effect towards Streptococcus aureus and Bacillus cereus (≤MIC 125 µg/mL). Vice versa, the conjugates with a cetyl substituent demonstrated the highest antibacterial activity in vitro towards S. aureus and B. cereus (MIC 16 µg/mL) but showed the lowest antituberculosis activity (MIC 12 µg/mL) among the compounds under study.

Similar content being viewed by others
REFERENCES
van Heijenoort, J., Nat. Prod. Rep., 2001, vol. 18, pp. 503–519.
Rani, C. and Khan, I.A., Eur. J. Pharm. Sci., 2016, vol. 83, pp. 62–70.
Derouaux, A., Sauvage, E., and Terrak, M., Front. Immunol., 2013, vol. 4, pp. 1–6.
Dumbre, S., Derouaux, A., Lescrinier, E., Piette, A., Joris, B., Terrak, M., and Herdewijn, P., J. Am. Chem. Soc., 2012, vol. 134, pp. 9343–9351.
Montoya-Peleaz, P.J., Riley, J.G., Szarek, W.A., Valvano, M.A., Schutzbach, J.S., and Brockhausen, I., Bioorg. Med. Chem. Lett., 2005, vol. 15, pp. 1205–1211.
Brockhausen, I., Larsson, E.A., and Hindsgaul, O., Bioorg. Med. Chem. Lett., 2008, vol. 18, pp. 804–807.
Riley, J.G., Xu, C., and Brockhausen, I., Carbohydr. Res., 2010, vol. 345, pp. 586–597.
Vinnikova, A.N., Torgov, V.I., Utkina, N.S., Veselovsky, V.V., Druzhinina, T.N., Wang, S., Brockhausen, I., and Danilov, L.L., Russ. J. Bioorg. Chem., 2015, vol. 41, pp. 105–107.
Li, Y., Zhou, Y., Mac, Y., and Li, X., Carbohydr. Res., 2011, vol. 346, pp. 1714–1720.
Cao, Z., Qu, Y., Zhou, J., Liu, W., and Yao, G., J. Carbohydr. Chem., 2015, vol. 34, pp. 28–40.
Gorityala, B.K., Lu, Z., Leow, M.L., Ma, J., and Liu, X.-W., J. Am. Chem. Soc., 2012, vol. 134, pp. 15229–15232.
Izmest’ev, E.S., Andreeva, O.V., Sharipova, R.R., Kravchenko, M.A., Garifullin, B.F., Strobykina, I.Yu., Kataev, V.E., and Mironov, V.F., Russ. J. Org. Chem., 2017, vol. 53, pp. 51–56.
Young, R.W., J. Am. Chem. Soc., 1952, vol. 74, pp. 1672–1673.
Filice, M., Guisan, J.M., Terreni, M., and Palomo, J.M., Nat. Protoc., 2012, vol. 7, pp. 1783–1796.
Fusari, M., Fallarini, S., Lombardi, G., and Lay, L., Bioorg. Med. Chem., 2015, vol. 23, pp. 7439–7447.
Chen, C., Liu, B., Xu, Y., Utkina, N., Zhou, D., Danilov, L., Torgov, V., Veselovsky, V., and Feng, L., Carbohydr. Res., 2016, vol. 430, pp. 36–43.
Saneyoshi, H., Yamamoto, Y., Kondo, K., Hiyoshi, Y., and Ono, A., J. Org. Chem., 2017, vol. 82, pp. 1796–1802.
Wang, S., Czuchry, D., Liu, B., Vinnikova, A.N., Gao, Y., Vlahakis, J.Z., Szarek, W.A., Wang, L., Feng, L., and Brockhausen, I., J. Bacteriol., 2014, vol. 196, pp. 3122–3133.
Chauviere, G., Bouteille, B., Enanga, B., de Albuquerque, C., Croft, S.L., Dumas, M., and Perie, J., J. Med. Chem., 2003, vol. 46, pp. 427–440.
Kyas, A. and Feigel, M., Helv. Chim. Acta, 2005, vol. 88, pp. 2375–2396.
Débieux, J.-L., Cosandey, A., Helgen, C., and Bochet, C.G., Eur. J. Org. Chem., 2007, pp. 2073–2077.
Bock, K., Guzman, J.F.-B., and Refn, S., Carbohydr. Res., 1992, vol. 232, pp. 353–357.
Chambers, D.J., Evans, G.R., and Fairbanks, A.J., Tetrahedron, 2004, vol. 60, pp. 8411–8419.
National Committee for Clinical Laboratory Standards, Methods for Dilution Antimicrobial Susceptibility, Tests for Bacteria That Grow Aerobically, Approved Standard, M7–A5, NCCLS, Wayne, Pa., USA, 2000, 6th ed.
National Committee for Clinical Laboratory Standards, Reference Method for Broth Dilution Antifungal Susceptibility Testing of Conidium–Forming Filamentous Fungi: Proposed Standard M38–P, NCCLS, Wayne, Pa., USA, 1998.
Kudoh, S. and Kudoh, T., Kekkaku, 1973, vol. 48, pp. 501–512.
ACKNOWLEDGMENTS
The study of antituberculosis activity of the compounds was supported by the Russian Science Foundation, project no. 14-50-00014. The authors gratefully acknowledge the Assigned Spectral–Analytical Center of FRC Kazan Scientific Center of RAS.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflict of interest. This article does not contain any studies involving animals or human participants performed by any of the authors.
Additional information
Translated by E. Shirokova
Abbreviations: GlcNAc, N-acetylglucosamine; GlmU-T, Gl-cNAc-1P-uridyl transferase; PG-T, PG glycosyl transferase; GlcNAc-1-P-alkyl, N-acetylglucosamine 1-alkylphosphate; MIC, minimal inhibitory concentration; PG, peptidoglycan; TMS-OTF, trimethylsilyl trifluoromethanesulfonate.
Corresponding author: phone: +7 (843) 273-9365; fax: +7 (843) 273-2253; e-mail: kataev@iopc.ru.
Rights and permissions
About this article
Cite this article
Sharipova, R.R., Garifullin, B.F., Sapunova, A.S. et al. Synthesis and Biological Activity of 3,4,-Tri-О-Acetyl-N-Acetylglucosamine and Tetraacetylglucopyranose Conjugated with Alkyl Phosphates. Russ J Bioorg Chem 45, 155–164 (2019). https://doi.org/10.1134/S1068162019020110
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1068162019020110