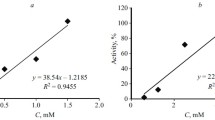
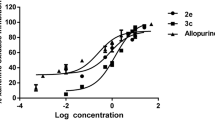
Abstract
New uracil and thymine derivatives, N1-,N3- and N1,N3-(RO-benzoyl)-(1H,3H)-pyrimidine- 2,4-diones, were synthesized (RO- is hydroxy, acetoxy- or methoxy-group). The compounds were studied in a complex of in vitro tests for the ability to inhibit the development of long-term complications of diabetes. Their ability to cleave cross-links of proteins has been evaluated. The most significant ways of pharmacological correction of thrombosis, angio-, nephro-, encephalo-, and cardiopathy, antiglycation, chelating, and antiplatelet activities, have been established. The most active compound in terms of antiplatelet action, N1- hydroxybenzoyluracil, exceeded acetylsalicylic acid by ~44%. In terms of their ability to chelate copper (II) cations, all compounds (with the exception of 1,3-bis(3-hydroxybenzoyl)-(1H,3H)-pyrimidine-2,4-dione that was not not studied in this test) showed the activity, whose IC50 fell in the range between that for pioglitazone (44.1 μM) and pyridoxamine (136.7 μM) comparison drugs. The best antiglycation effect at the 1 mM concentration was observed for N1,N3-bismethoxy- and N1,N3-bisacetoxybenzoyl derivatives of thymine. The maximum activity to cleave cross-links of proteins (C = 1 mM), comparable to that of alagebrium, was established for 1,3-bis(4-methoxybenzoyl)uracil, for which also high rates of other estimated activities were noted. Thus, the N1-,N3- and N1,N3-(RO-benzoyl) derivatives of uracil and thymine are promising basiсs for creating drugs that suppress the development of long-term complications of diabetes.
Similar content being viewed by others
Abbreviations
- DM:
-
diabetes mellitus
- ROS:
-
reactive oxygen species
- GEP:
-
glycation-end products
- NMR:
-
nuclear magnetic resonance
- BSA:
-
bovine serum albumin
References
Dedov, I.I., Omel’yanovskii, V.V., Shestakova, M.V., Avksent’eva, M.V., and Ignat’eva, V.I., Sakharnyi Diabet, 2016, vol. 19, no. 1, pp. 30–43.
Dedov, I.I., Kontsevaya, A.V., Shestakova, M.V., Belousov, Yu.B., Balanova, Yu.A., Khudyakov, M.B., and Karpov, O.I., Sakharnyi Diabet, 2016, no. 119(6), pp. 518–527.
Petrov, V.I., Spasov, A.A., Cheplyaeva, N.I., and Lenskaya, K.V., Antidiabetogennyi potentsial benzimidazolov: khimiya, farmakologiya, klinika (Antidiabetic Potential of Benzimidazoles: Chemistry, Pharmacology, and Clinical Use), Spasov, A.A, Petrov, V.I, and Minkin, M.I., Eds., Volgograd: Vogograd. Gos. Univ., 2016, chapter 1.
Matough, F.A., Budin, S.B., Hamid, Z.A., Alwahaibi, N., and Mohamed, J., Sultan Qaboos Univ. Med. J., 2012, vol. 12, no. 1, pp. 5–18.
Ullaha, A., Khana, A., and Khan, I., Saudi Pharm. J., 2016, vol. 24, pp. 547–553.
Deroo, S., Stengel, F., Mohammadi, A., Henry, N., Hubin, E., Krammer, E.M., Aebersold, R., and Raussens, V., ACS Chem. Biol., 2015, vol. 10, no. 4, pp. 1010–1016.
Sell, D.R., Strauch, C.M., Shen, W., and Monnier, V., Biochem. J., 2007, vol. 404, pp. 269–277.
Nagai, R., Murray, D.B., Metz, T.O., and Baynes, J.W., Diabetes, 2012, vol. 61, no. 3, pp. 549–559.
Spasov, A.A., Cheplyaeva, N.I., and Snigur, G.L., Antidiabetogennyi potentsial benzimidazolov: khimiya, farmakologiya, klinika (Antidiabetic Potential of Benzimidazoles: Chemistry, Pharmacology, and Clinical Use), Spasov, A.A, Petrov, V.I, and Minkin, M.I., Eds., Volgograd: Vogograd. Gos. Univ., 2016, chapter 9.
Kawada, H. and Kador, P.F., J. Med Chem., 2015, vol. 58, no. 22, pp. 8796–8805.
Hess, K. and Grant, P.J., Thromb. Haemost., 2011, vol. 1, pp. 43–54.
Spasov, A.A., Babkov, D.A., Sysoeva, V.A., Litvinov, R.A., Shamshina, D.D., Ulomsky, E.N., Savateev, K.V., Fedotov, V.V., Slepukhin, P.A., Chupakhin, O.N., Charushin, V.N., and Rusinov, V.L., Archiv der Pharmazie, 2017, vol. 350, no. 12.
Stocker, R., Curr. Opin. Lipidol., 2009, vol. 20, no. 3, pp. 227–235.
Koo, Y.K., Kim, J.M., Koo, J.Y., Kang, S.S., Bae, K., Kim, Y.S., Chung, J.H., and Yun-Choi, H.S., Pharmazie, 2010, vol. 65, pp. 624–628.
Bruno, O., Schenone, S., Ranise, A., Bondavalli, F., Barocelli, E., Ballabeni, V., Chiavarini, M., Bertoni, S., Tognolini, M., and Impicciatore, M., Bioorg. Med. Chem., 2001, vol. 9, pp. 629–636.
Esfahanizadeh, M., Mohebbi, S., Bozorg, B.D., Amidi, S., Gudarzi, A., Ayatollahi, S.A., and Kobarfard, F., Iran. J. Pharm. Res., 2015, vol. 14, no. 2, pp. 417–427.
Spasov, A.A., Kucheryavenko, A.F., and Lenskaya, K.V., Antidiabetogennyi potentsial benzimidazolov: khimiya, farmakologiya, klinika (Antidiabetic Potential of Benzimidazoles: Chemistry, Pharmacology, and Clinical Use), Spasov, A.A, Petrov, V.I, and Minkin, M.I., Eds., Volgograd: Vogograd. Gos. Univ., 2016, chapter 10.
Segovia, A.S.R., Wrobel, K., Aguilar, F.J.A., Escobosa, A.R.C., and Wrobel, K., Metallomics, 2017, vol. 9, no. 2, pp. 132–140.
Hernández, B., Pflüger, F., Kruglik, S.G., Cohen, R., and Ghomi, M., J. Pharm. Biomed. Anal., 2015, vol. 114, pp. 42–48.
Marunaka, Y., World J. Diabetes, 2015, vol. 6, no. 1, pp. 125–135.
Wolff, S.P. and Dean, R.T., Biochem. J., 1987, vol. 245, no. 1, pp. 243–250.
Brel’, A.K., Lisina, S.V., and Popov, S.S., RF Patent no. 2601309, 2016.
Brel’, A.K., Lisina, S.V., Budaeva, Yu.N., and Popov, S.S., Zh. Obshch. Khim., 2015, vol. 85, no. 9, pp. 1561–1563.
Brel’, A.K., Spasov, A.A., Lisina, S.V., Popov, S.S., and Rashchenko, A.I., RF Patent no. 2643520, 2018.
Brel’, A.K., Lisina, S.V., Popov, S.S., and Budaeva, Yu.N., Zh. Obshch. Khim., 2016, vol. 86, no. 3, pp. 549–551.
Shamshina, D.D. and Litvinov, R.A., Vestn. Volg. Gos. Med. Univ., 2018, no. 1 (65), pp. 115–117.
Ivanov, A.V., Shamshina, D.D., Litvinov, R.A., and Batrakov, V.V., Vestn. Volg. Gos. Med. Univ., 2018, no. 2 (66), pp. 47–49.
Spasov, A.A., Zhukovskaya, O.N., Brigadirova, A.A., Abbas, H.S.A., Anisimova, V.A., Sysoeva, V.A., Rashchenko, A.I., Litvinov, R.A., Mayka, O.Yu., Babkov, D.A., and Morkovnikc, A.S., Russ. Chem. Bull., 2017, vol. 66, pp. 1905–1912.
Valdés-Ramos, R., Guadarrama-López, A.L., Martínez-Carrillo, B.E., and Benítez-Arciniega, A.D., Endocr. Metab. Immune Disord. Drug Targets, 2015, vol. 15, no. 1, pp. 54–63.
Golbidi, S., Badran, M., and Laher, I., Front. Pharmacol., 2011, vol. 2, p. 69.
Li Sun et al., RF Patent no. 2008134899/04, 2007.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Spasov, A.A., Brel, A.K., Litvinov, R.A. et al. Evaluation of N-Hydroxy-, N-Metoxy-, and N-Acetoxybenzoyl-Substituted Derivatives of Thymine and Uracil as New Substances for Prevention and Treatment of Long-Term Complications of Diabetes Mellitus. Russ J Bioorg Chem 44, 769–777 (2018). https://doi.org/10.1134/S1068162019010163
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1068162019010163