Abstract
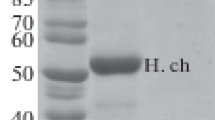
Rabies is a zoonotic disease, for which effective treatment methods after the onset of clinical symptoms have not been developed yet. Polyclonal sera, both human and equine, along with vaccines are important means of disease prophylaxis. However, due to adverse reactions to the immunoglobulins of animal origin, high cost, and limited availability of the safer human serum, polyclonal antibodies should be substituted for a stable and efficient preparation, which is recombinant neutralizing antirabies monoclonal antibodies (mAbs). This paper reports generation of the humanized mAb 1C5, which binds with the antigenic site (AS) III of the rabies virus glycoprotein (RABVG) and demonstrates high virus neutralization activity in the fluorescent antibody virus neutralization test, as a result of expression in the Chinese hamster ovary (CHO) cells.
Similar content being viewed by others
Abbreviations
- CHO:
-
Chinese hamster ovary cells
- ERIG:
-
equine rabies immunoglobulin
- FAVN:
-
fluorescent antibody virus neutralization test
- HRIG:
-
human rabies immunoglobulin
- PBS:
-
phosphate-buffered saline
- RABV:
-
rabies virus
- RABVG:
-
rabies virus glycoprotein
- RIG:
-
rabies immunoglobulin
- SOE-PCR:
-
splicing by overlap extension polymerase chain reaction
- VH and VL:
-
variable domains of immunoglobulin heavy and light chains
- AS:
-
antigenic site
- mAb:
-
monoclonal antibodies
- HP:
-
horseradish peroxidase
- PEP:
-
post-exposure prophylaxis
- EPR:
-
endoplasmic reticulum
References
Fooks, A.R., Banyard, A.C., Horton, D.L., Johnson, N., McElhinney, L.M., and Jackson, A.C., Lancet, 2014, vol. 11, pp. 1389–1399.
Fisher, C.R., Streicker, D.G., and Schnell, M.J., Nat. Rev. Microbiol., 2018, vol. 16, pp. 241–255.
Lafon, M., J. Neurovirol., 2005, vol. 11, pp. 82–87.
Kuzmina, N.A., Kuzmin, I.V., Ellison, J.A., and Rupprecht, Ch.E., J. Antivir. Antiretrovir., 2013, vol. 5, pp. 37–43.
Crucell Holland, B.V., A Randomized Phase II Trial to Compare the Safety and Neutralizing Activity of CL184 in Combination with Rabies Vaccine vs. HRIG or Placebo in Combination with Rabies Vaccine in Healthy Adult Subjects, 2011. https://clinicaltrials.gov/show/NCT00656097.
Crucell Holland, B.V., Randomized Phase II Trial on Safety and Neutralizing Activity of CL184 and Rabies Vaccine Versus Human Rabies Immune Globulin (HRIG) and Rabies Vaccine in Children and Adolescents, 2012. https://clinicaltrials.gov/show/NCT00708084.
Crucell Holland, B.V., Rabies Virus Neutralizing Activity and Safety of CL184, a Monoclonal Antibody Cocktail, in Simulated Rabies Post–Exposure Prophylaxis in Healthy Adults, 2013. https://clinicaltrials. gov/ct2/show/study/NCT01228383.
Dietzschold, B., Gore, M., Casali, P., Ueki, Y., Rupprecht, C.E., Notkins, A.L., and Koprowski, H., J. Virol., 1990, vol. 64, pp. P. 3087–3090.
Kramer, R.A., Marissen, W.E., Goudsmit, J., Visser, T.J., Clijsters-Van der Horst, M., Bakker, A.Q., de Jong, M., Jongeneelen, M., Thijsse, S., Backus, H.H., Rice, A.B., Weldon, W.C., Rupprecht, C.E., Dietzschold, B., Bakker, A.B., and de Kruif, J., Eur. J. Immunol., 2005, vol. 35, pp. 2131–2145.
Sloan, S.E., Hanlon, C., Weldon, W., Niezgoda, M., Blanton, J., Self, J., Rowley, K.J., Mandell, R.B., Babcock, G.J., Thomas, W.D., Rupprecht, C.E., and Ambrosino, D.M., Vaccine, 2007, vol. 25, pp. 2800–2810.
Serum Institute of India Ltd. A Phase II/III, Randomized, Multi-Centric, Comparator-controlled Study of the Safety and Neutralizing Activity of a Human Monoclonal Antibody to Rabies (SII RMAb) Administered in Conjunction with Rabies Vaccine for Postexposure Prophylaxis in Patients following Potential Rabies Exposure, CTRI/2012/05/002709, 2012. http://www.ctri.nic.in/Clinicaltrials/pmaindet2.php? trialid=4191.
Benevolenskii, S.V., Zatsepin, S.S., Klyachko, E.V., Morozkina, E.V., Pozdnyakova, L.P., Sveshnikov, P.G., Solopova, O.N., Shemchukova, O.B., and Yagudin, O.B., Humanized antigen binding fragments (Fab) against the rabies virus, an isolated DNA fragment encoding Fab against the rabies virus, an yeast cell transformed with the DNA fragment, and a method for producing Fab against the rabies virus using yeast, RF Patent no. RU2440412 (C2), 2012.
Sveshnikov, P.G., Yagudin, T.A., Morozkina, E.V., Klyachko, E.V., Zatsepin, S.S., Benevolenskii, S.V., Shemchukova, O.B., Pozdnyakova, L.P., and Solopova, O.N., Vestn. Mosk. Univ., 2010, vol. 51, pp. 185–190.
Drew, P.D., Moss, M.T., Pasieka, T.J., Grose, C., Harris, W.J., and Porter, A.J., J. Gen. Virol., 2001, vol. 82, pp. 1959–1963.
Hultberg, A., Temperton, N.J., Rosseels, V., Koenders, M., Gonzalez-Pajuelo, M., Schepens, B., Itati, IbanezL., Vanlandschoot, P., Schillemans, J., Saunders, M., Weiss, R.A., Saelens, X., Melero, JoseA., Verrips, C.Th., Gucht, S.V., and de Haard, H.J., PLoS One, 2011, vol. 6. e17665.
Tillib, S.V., Ivanova, T.I., Vasil’ev, L.A., Metlin, A.E., Logunov, D.Yu., Tutykhina, I.L., Alekseeva, S.V., Naroditskii, B.S., and Gintsburg, A.L., Trimerized single- domain antibody specifically binding to glycoprotein g of the rabies virus, which neutralizes the rabies virus, RF Patent no. RU2533802 (C1), 2014.
Schlatter, S., Stansfield, S.H., Dinnis, D.M., Racher, A.J., Birch, J.R., and James, D.C., Biotechnol. Prog., 2005, vol. 21, pp. 122–133.
Salfeld, J.G., Nat. Biotechnol., 2007, vol. 25, pp. 1369–1372.
Schroeder, H.W., Cavacini, Jr., and Cavacini, L., J Allergy Clin Immunol., 2010, vol. 125, pp. 41–52.
Jefferis, R., Expert Opin. Biol. Ther., 2007, vol. 7, pp. 1401–1413.
Alyanakian, M.A., Bernatowska, E., Scherrmann, J.M., Aucouturier, P., and Poplavsky, J.L., Vox Sang, 2003, vol. 84, pp. 188–192.
Birch, J.R. and Racher, A.J., Adv. Drug Deliv. Rev., 2006, vols. 5–6, pp. 671–685.
de Kruif, J., Bakker, A.B., Marissen, W.E., Kramer, R.A., Throsby, M., Rupprecht, C.E., and Goudsmit, J., Annu. Rev. Med., 2007, vol. 58, pp. 359–368.
Gaudin, Y., Ruigrok, R.W., Tuffereau, C., Knossow, M., and Flamand, A., Virology, 1992, vol. 187, pp. 627–632.
Lafon, M., Wiktor, T.J., and Macfarlan, R.I., J. Gen. Virol., 1983, vol. 64, pp. 843–851.
Muller, T., Dietzschold, B., Ertl, H., Fooks, A.R., Freuling, C., Fehlner-Gardiner, C., Kliemt, J., Meslin, F.X., Franka, R., Rupprecht, C.E., Tordo, N., Wanderler, A.I., and Kieny, M.P., PLoS Negl. Trop. Dis., 2009, vol. 3, e542.
Benmansour, A., Leblois, H., Coulon, P., Tuffereau, C., Gaudin, Y., Flamand, A., and Lafay, F., J. Virol., 1991, vol. 65, pp. 4198–4203.
De Benedictis, P., Minola, A., Rota, NodariE., Aiello, R., Zecchin, B., Salomoni, A., Foglierini, M., Agatic, G., Vanzetta, F., Lavenir, R., Lepelletier, A., Bentley, E., Weiss, R., Cattoli, G., Capua, I., Sallusto, F., Wright, E., Lanzavecchia, A., Bourhy, H., and Corti, D., EMBO Mol. Med., 2016, vol. 8, pp. 407–421.
Laemmli, U.K., Nature, 1970, vol. 227, pp. 680–685.
Friguet, B., Chaffotte, A.F., Djavadi-Ohaniance, L., and Goldberg, M.E., J. Immunol. Meth., 1985, vol. 77, pp. 305–319.
Klotz, I.M., Proteins, 1953, vol. 1, pp. 727–806.
Tijssen, P. and Kurstak, E., Anal. Biochem., 1984, vol. 136, pp. 451–457.
International Office of Epizootics. Biological Standards Commission, International Office of Epizootics. International Committee, Manual of Diagnostic Tests and Vaccines for Terrestrial Animals: Mammals, Birds and Bees, Office International des Épizooties, 2008, vol. 2.
WHO Consultation on a Rabies Monoclonal Antibody Cocktail for Rabies Post Exposure Treatment, Geneva: WHO, 2002.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ilina, E.N., Solopova, O.N., Balabashin, D.S. et al. Generation and Characterization of a Neutralizing Monoclonal Antibody Against Rabies Virus. Russ J Bioorg Chem 44, 695–704 (2018). https://doi.org/10.1134/S1068162019010072
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1068162019010072