Abstract
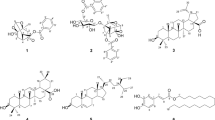
Spiraea L. belongs to a genus of deciduous-leaved shrubs in the Rosaceae family that is abundant in Eastern Siberia. The study of six species of Spiraea growing in the Baikal region revealed that they were characterized with high content of phenolic compounds and their extracts demonstrated an inhibitory effect on α-amylase. It was established using correlation analysis that flavonoids were the leading components associated with this biological effect of the extracts. The extracts from S. salicifolia leaves demonstrated the highest activity with IC50 69.30 μg/mL. Fractionation and chromatographic separation of the extracted compounds from the ethyl acetate fraction of S. salicifolia allowed isolation of 18 compounds with 15 of those isolated for the first time from this species including trifolin, 6′′-O-caffeoyl-hyperoside, 6′′-O-caffeoylisoquercitrin, 6′′-O-caffeoyl-astragalin, 1-O-p-hydroxybenzoyl-6-O-p-coumaroyl-β-d-glucopyranoside, 3,4,5-tri-O-caffeoylquinic acid, isoramnetinin-3-O-β-d-glucopyranoside, tiliroside, isoramnetin-3-O-α-lrhamnopyranoside, 1-O-cis-cinnamoyl-6-O-(2′-methylene-4′-hydroxybutyroyl)-β-d-glucopyranose, 1-О-(4′′-hydroxy-3′′-methylfurane-2′′-one)-6-О-trans-cinnamoyl-β-d-glucopyranose, 1-О-(4′′-hydroxy-3′′-methylfurane-2′′-one)-6-О-cis-cinnamoyl-β-d-glucopyranose, 6-tuliposide A, and tulipalin A. Flavonoid caffeoyl glycosides were identified as the most active inhibitors of α-amylase with 6′′-O-caffeoyl-hyperoside demonstrating the maximum IC50 of 46.18 μg/mL that determined total anti-α-amylase effect of the S. salicifolia extract. The total content of 6′′-О-caffeoyl-hyperoside in S. salicifolia leaves was 0.60–10.53 mg/g and of flavonoids, 12.02–23.17 mg/g. The study showed that the acylated flavonoids from Spiraea were effective inhibitors of α-amylase.
Similar content being viewed by others
References
Etxeberria, U., Garza, A.L., Campion, J., Martinez, J.A., and Milagro, F.I., Antidiabetic effects of natural plant extracts via inhibition of carbohydrate hydrolysis enzymes with emphasis on pancreatic alpha amylase, Expert Opin. Ther. Targets, 2012, vol. 16, pp. 1–29.
Mathers, C.D. and Loncar, D., Projections of global mortality and burden of disease from 2002 to 2030, PLoS Med., 2006, vol. 3, pp. 2011–2030.
Yin, Z., Zhang, W., Feng, F., Zhang, Y., and Kang, W., α-Glycosidase inhibitors isolated from medicinal plants, Food Sci. Hum. Wellness, 2014, vol. 3, pp. 136–174.
Xiao, J., Ni, X., Kai, G., and Chen, X., A review on structure-activity relationship of dietary polyphenols inhibiting α-amylase, Crit. Rev. Food Sci. Nutr., 2013, vol. 53, pp. 497–506.
Playford, R.J., Pither, C., Gao, R., and Middleton, S.J., Use of the alpha-glucosidase inhibitor acarbose in patients with ‘Middleton syndrome’: Normal gastric anatomy but with accelerated gastric emptying causing postprandial reactive hypoglycemia and diarrhea, Can. J. Gastroenterol., 2013, vol. 27, pp. 403–404.
Sales, P.M., Souza, P.M., Simeoni, L.A., Magalhaes, P.O., and Silveira, D., α-Amylase inhibitors: A review of raw material and isolated compounds from plant source, J. Pharm. Pharmaceut. Sci., 2012, vol. 15, pp. 141–183.
Ochir, S., Nishizawa, M., and Park, B.J., Inhibitory effect of Rosa gallica on the digestive enzymes, J. Nat. Med., 2010, vol. 64, pp. 175–280.
Grussu, D., Stewart, D., and McDougall, G.J., Berry polyphenols inhibit alpha-amylase in vitro: identifying acting components in rowanberry and raspberry, J. Agric. Food Chem., 2011, vol. 59, pp. 2324–2331.
Hamdan, I.I. and Afifi, F.U., Screening of Jordanian flora for alpha-amylase inhibitory activity, Pharm. Biol, 2008, vol. 46, pp. 746–750.
Olennikov, D.N., Kashchenko, N.I., and Chirikova, N.K., Meadowsweet teas as new functional beverages: Comparative analysis of nutrients, phytochemicals and biological effects of four Filipendula species, Molecules, 2017, vol. 22, art. 16. doi 10.3390/molecules22010016
Kashchenko, N.I., Chirikova, N.K., and Olennikov, D.N., Agrimoniin, an active ellagitannin from Comarum palustre herb with anti-α-glucosidase and antidiabetic potential in streptozotocin-induced diabetic rats, Molecules, 2017, vol. 22, art. 75. doi 10.3390/molecules22010073
Chirikova, N.K., Olennikov, D.N., and Tankhaeva, L.M., Quantitative determination of flavonoid content in the aerial part of Baical scullcap (Scutellaria baicalensis Georgi), Russ. J. Bioorg. Chem., 2010, vol. 36, pp. 915–922.
Sun, B., Ricardo-da-Silva, J.M., and Spranger, I., Critical factors of vanillin assay for catechins and proanthocyanidins, J. Agric. Food Chem., 1998, vol. 46, pp. 4267–4274.
Galvez, M., Martin-Cordero, C., Houghton, P.J., and Ayuso, M.J., Antioxidant activity of methanol extracts obtained from Plantago species, J. Agric. Food Chem., 2005, vol. 53, pp. 1927–1933.
Olennikov, D.N. and Kashchenko, N.I., Componential profile and amylase inhibiting activity of phenolic compounds from Calendula officinalis L. leaves, Sci. World J., 2014, vol. 2014, art. ID 654193. doi 10.1155/2014/654193
Olennikov, D.N., Stolbikova, A.V., Tankhaeva, L.M., and Petrov, E.V., Phenylpropanoids and polysaccharides of Plantago depressa and P. media, Chem. Nat. Comp., 2011, vol. 47, pp. 165–169.
Azimova, S.S. and Vinogradova, V.I., Natural Compounds. Flavonoids: Plant Sources, Structure and Properties, New York: Springer, 2013.
Yoshida, K., Hishida, A., Iida, O., Hosokawa, K., and Kawabata, J., Flavonol caffeoylglycosides as α-glucosidase inhibitors from Spiraea cantoniensis flowers, J. Agric. Food Chem., 2008, vol. 56, pp. 4367–4371.
Choudhary, M.I., Naheed, N., Abbaskhan, A., Ali, S., and Atta-ur-Rahman, Hemuterpene glucosides and other constituents from Spiraea canescens, Phytochemistry, 2009, vol. 70, pp. 1467–1473.
Hiradate, S., Morita, S., Sugie, H., Fijii, Y., and Harada, J., Phytotoxic cis-cinnamoyl glycosides from Spiraea thunbergii, Phytochemistry, 2004, vol. 65, pp. 731–739.
Kim, C.-S., Datta, P.K., Hara, T., Itoh, E., and Horiike, M., Precursor of α-methylene-γ-butyrolactone involved in the insecticidal activity of Thunberg spiraea, Spiraea thunbergii, Biosci. Biotechnol. Biochem., 1999, vol. 63, pp. 152–154.
Ahn, B., Oh, K., Park, S., Chung, S., Cho, E., Kim, J., Ro, J., and Lee, K., Phenolic compounds from leaves of Spiraea salicifolia, Korean J. Pharmacogn., 1996, vol. 27, pp. 178–183.
Krivosheev, I.M., Pharmacognostic study of willowleaf meadowsweet (Spiraea salicifolia L.) growing in Eastern Siberia, Extended Abstract of Cand. Sci. (Farm.) Dissertation, Ulan-Ude, 2014.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © N.I. Kashchenko, N.K. Chirikova, D.N. Olennikov, 2017, published in Khimiya Rastitel’nogo Syr’ya, 2017, No. 4, pp. 81–90.
Rights and permissions
About this article
Cite this article
Kashchenko, N.I., Chirikova, N.K. & Olennikov, D.N. Acylated Flavonoids from Spiraea Genus as Inhibitors of α-Amylase. Russ J Bioorg Chem 44, 876–886 (2018). https://doi.org/10.1134/S1068162018070051
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1068162018070051